Page 177 - Introduction to Colloid and Surface Chemistry
P. 177
166 The solid-liquid interface
The action of the detergent is to lower -y DW and y sw, thus decreasing
and increasing the ease with which the dirt particle can be
W SD
detached by mechanical agitation.
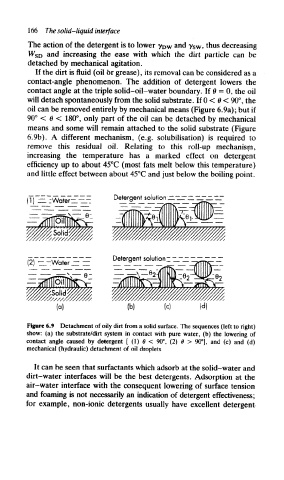
If the dirt is fluid (oil 6r grease), its removal can be considered as a
contact-angle phenomenon. The addition of detergent lowers the
contact angle at the triple solid-oil-water boundary. If B = 0, the oil
will detach spontaneously from the solid substrate. If 0 < 6 < 90°, the
oil can be removed entirely by mechanical means (Figure 6.9a); but if
90° < 0 < 180°, only part of the oil can be detached by mechanical
means and some will remain attached to the solid substrate (Figure
6.9b), A different mechanism, (e.g. solubilisation) is required to
remove this residual oil. Relating to this roll-up mechanism,
increasing the temperature has a marked effect on detergent
efficiency up to about 45°C (most fats melt below this temperature)
and little effect between about 45°C and just below the boiling point.
Detergent solution
(]} HI :Waterz_~z_~:
~7~"L~~ Detergent solution~ z_~_z~
(2) n;~Water jz
Figure 6.9 Detachment of oily dirt from a solid surface. The sequences (left to right)
show: (a) the substrate/dirt system in contact with pure water, (b) the lowering of
contact angle caused by detergent [ (1) 0 < 90°, (2) 8 > 90°], and (c) and (d)
mechanical (hydraulic) detachment of oil droplets
It can be seen that surfactants which adsorb at the solid-water and
dirt-water interfaces will be the best detergents. Adsorption at the
air-water interface with the consequent lowering of surface tension
and foaming is not necessarily an indication of detergent effectiveness;
for example, non-ionic detergents usually have excellent detergent