Page 181 - Introduction to Colloid and Surface Chemistry
P. 181
170 The solid-liquid interface
in general, more complicated than that of gas adsorption, since
adsorption from solution always involves competition between
solute(s) and solvent or between the components of a liquid mixture
for the adsorption sites. Consider, for example, a binary liquid
mixture in contact with a solid. Zero adsorption refers to uniform
mixture composition right up to the solid surface, even though
(unlike zero gas adsorption) both components are, in fact, present at
the solid surface. If the proportion of one of the components at the
surface is greater than its proportion in bulk, then that component is
positively adsorbed and, consequently, the other component is
negatively adsorbed. Apparent, rather than true, adsorption isotherms
are, therefore, calculated from changes in solution concentration.
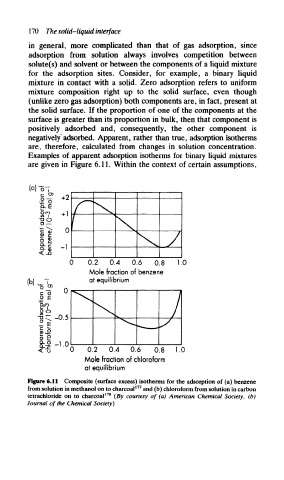
Examples of apparent adsorption isotherms for binary liquid mixtures
are given in Figure 6.11. Within the context of certain assumptions,
foj-57
C CD
•2-0
0-E
+ 1
Z
Q- c
-5 -1
0 0.2 0.4 0.6 0.8 1.0
Mole fraction of benzene
(M 7 at equilibrium
""ft O>
2-o o __ — | I
O £ s
^ v s
OJ*> X.
/
-8 ^ -0.5 X x y /
^NS. */
ll *^«— •
0 2
a o
-1.0
0.2 0.4 0.6 0.8 1.0
Mole fraction of chloroform
at equilibrium
Figure 6.11 Composite (surface excess) isotherms for the adsorption of (a) benzene
177
from solution in methanol on to charcoal and (b) chloroform from solution in carbon
tetrachloride on to charcoal 178 (By courtesy of (a) American Chemical Society, (b)
Journal of the Chemical Society)