Page 182 - Introduction to Colloid and Surface Chemistry
P. 182
The solid-liquid interface 171
the individual adsorption isotherms can be calculated from the
apparent (or composite) adsorption isotherm together with appro-
63
priate vapour adsorption data .
Adsorption from solution behaviour can often be predicted
qualitatively in terms of the polar/non-polar nature of the solid and of
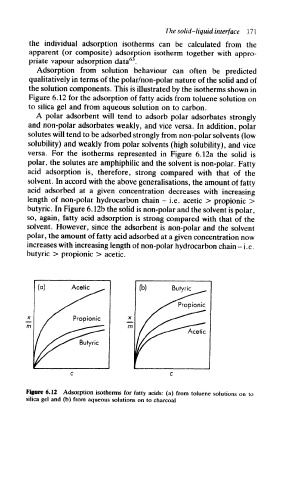
the solution components. This is illustrated by the isotherms shown in
Figure 6.12 for the adsorption of fatty acids from toluene solution on
to silica gel and from aqueous solution on to carbon.
A polar adsorbent will tend to adsorb polar adsorbates strongly
and non-polar adsorbates weakly, and vice versa. In addition, polar
solutes will tend to be adsorbed strongly from non-polar solvents (low
solubility) and weakly from polar solvents (high solubility), and vice
versa. For the isotherms represented in Figure 6.12a the solid is
polar, the solutes are amphiphilic and the solvent is non-polar. Fatty
acid adsorption is, therefore, strong compared with that of the
solvent. In accord with the above generalisations, the amount of fatty
acid adsorbed at a given concentration decreases with increasing
length of non-polar hydrocarbon chain -i.e. acetic > propionic >
butyric. In Figure 6.12b the solid is non-polar and the solvent is polar,
so, again, fatty acid adsorption is strong compared with that of the
solvent. However, since the adsorbent is non-polar and the solvent
polar, the amount of fatty acid adsorbed at a given concentration now
increases with increasing length of non-polar hydrocarbon chain - i.e.
butyric > propionic > acetic.
Figure 6.12 Adsorption isotherms for tatty acids: (a) from toluene solutions on to
silica gel and (b) from aqueous solutions on to charcoal