Page 52 - Introduction to Colloid and Surface Chemistry
P. 52
42 Kinetic properties
(b)
Solution \ Solvent
Membrane
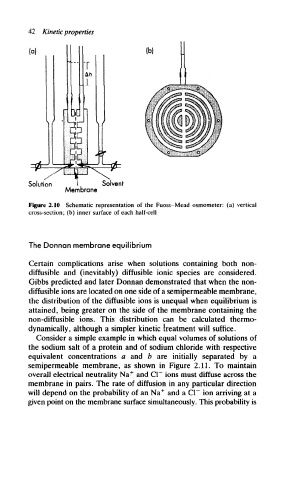
Figure 2.10 Schematic representation of the Fuoss-Mead osmometer: (a) vertical
cross-section; (b) inner surface of each half-cell
The Donnan membrane equilibrium
Certain complications arise when solutions containing both non-
diffusible and (inevitably) diffusible ionic species are considered.
Gibbs predicted and later Donnan demonstrated that when the non-
diffusible ions are located on one side of a semipermeable membrane,
the distribution of the diffusible ions is unequal when equilibrium is
attained, being greater on the side of the membrane containing the
non-diffusible ions. This distribution can be calculated thermo-
dynamically, although a simpler kinetic treatment will suffice.
Consider a simple example in which equal volumes of solutions of
the sodium salt of a protein and of sodium chloride with respective
equivalent concentrations a and b are initially separated by a
semipermeable membrane, as shown in Figure 2.11. To maintain
overall electrical neutrality Na + and Cl~~ ions must diffuse across the
membrane in pairs. The rate of diffusion in any particular direction
will depend on the probability of an Na + and a Cl~ ion arriving at a
given point on the membrane surface simultaneously. This probability is