Page 104 - Introduction to Paleobiology and The Fossil Record
P. 104
PALEOECOLOGY AND PALEOCLIMATES 91
(Fig. 4.12b). The Zechstein benthos was dom-
inated by diverse associations of brachiopods, Sea level
overshadowed in the higher tiers by fan- and
vase-shaped bryozoans (Hollingworth & Pet-
tigrew 1988). Both groups were sessile fi lter sand
feeders. Stalked echinoderms were rarer and oxic mud
occupied the highest tiers. Mollusks such as Factors affecting benthos anoxic mud
bivalves and gastropods were important light
deposit feeders and grazers. One of the largest
predators was Janassa, a benthic ray, equipped oxygen
with a formidable battery of teeth capable of food
crushing the shells of the sedentary benthos.
salinity
Megaguilds substrate
Assignment of organisms to megaguilds pro- tidal shoals Substrate mobility
vides another way to classify and understand
the components of a fossil community. Guilds turbidity
are groups of functionally similar organisms
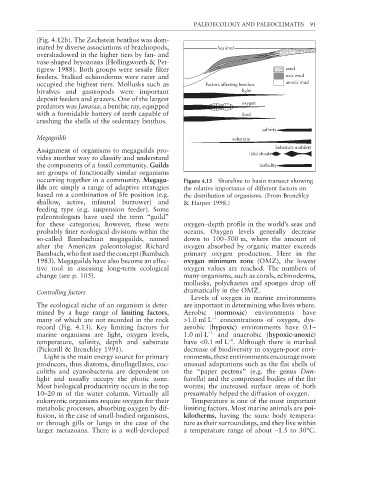
occurring together in a community. Megagu- Figure 4.13 Shoreline to basin transect showing
ilds are simply a range of adaptive strategies the relative importance of different factors on
based on a combination of life position (e.g. the distribution of organisms. (From Brenchley
shallow, active, infaunal burrower) and & Harper 1998.)
feeding type (e.g. suspension feeder). Some
paleontologists have used the term “guild”
for these categories; however, these were oxygen–depth profile in the world’s seas and
probably finer ecological divisions within the oceans. Oxygen levels generally decrease
so-called Bambachian megaguilds, named down to 100–500 m, where the amount of
after the American paleontologist Richard oxygen absorbed by organic matter exceeds
Bambach, who first used the concept (Bambach primary oxygen production. Here in the
1983). Megaguilds have also become an effec- oxygen minimum zone (OMZ), the lowest
tive tool in assessing long-term ecological oxygen values are reached. The numbers of
change (see p. 105). many organisms, such as corals, echinoderms,
mollusks, polychaetes and sponges drop off
Controlling factors dramatically in the OMZ.
Levels of oxygen in marine environments
The ecological niche of an organism is deter- are important in determining who lives where.
mined by a huge range of limiting factors, Aerobic (normoxic) environments have
−1
many of which are not recorded in the rock >1.0 ml L concentrations of oxygen, dys-
record (Fig. 4.13). Key limiting factors for aerobic (hypoxic) environments have 0.1–
−1
marine organisms are light, oxygen levels, 1.0 ml L and anaerobic (hypoxic-anoxic)
−1
temperature, salinity, depth and substrate have <0.1 ml L . Although there is marked
(Pickerill & Brenchley 1991). decrease of biodiversity in oxygen-poor envi-
Light is the main energy source for primary ronments, these environments encourage more
producers, thus diatoms, dinofl agellates, coc- unusual adaptations such as the fl at shells of
coliths and cyanobacteria are dependent on the “paper pectens” (e.g. the genus Dun-
light and usually occupy the photic zone. barella) and the compressed bodies of the fl at
Most biological productivity occurs in the top worms; the increased surface areas of both
10–20 m of the water column. Virtually all presumably helped the diffusion of oxygen.
eukaryotic organisms require oxygen for their Temperature is one of the most important
metabolic processes, absorbing oxygen by dif- limiting factors. Most marine animals are poi-
fusion, in the case of small-bodied organisms, kilotherms, having the same body tempera-
or through gills or lungs in the case of the ture as their surroundings, and they live within
larger metazoans. There is a well-developed a temperature range of about −1.5 to 30°C.