Page 24 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 24
Introduction to Hydrogen Technology 13
1.2.1. The different fuel cell technologies
Several types of fuel cells are currently being researched. The
classification of fuel cells is generally according to the nature of the
electrolyte because it determines, on the one hand, the temperature at which
the battery operates and, on the other hand, the ion type ensuring the ionic
conduction [BLU 07]. For example:
– Polymer-Electrolyte Membrane Fuel Cell or Proton-Exchange
Membrane Fuel Cell ~80°C (PEMFC);
– Direct Methanol Fuel Cell ~60–100°C (DMFC);
– Alkaline Fuel Cell ~100°C (AFC);
– Phosphoric Acid Fuel Cell ~200°C (PAFC);
– Molten Carbonate Fuel Cell ~700°C (MCFC);
– Solide Oxyde Fuel Cell ~800–1,000°C (SOFC).
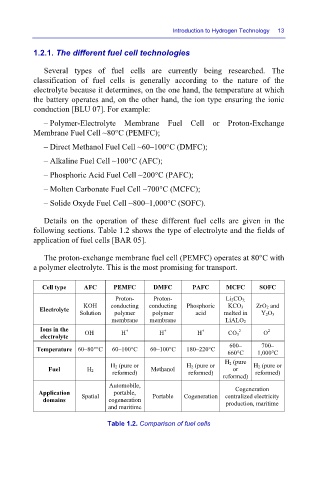
Details on the operation of these different fuel cells are given in the
following sections. Table 1.2 shows the type of electrolyte and the fields of
application of fuel cells [BAR 05].
The proton-exchange membrane fuel cell (PEMFC) operates at 80°C with
a polymer electrolyte. This is the most promising for transport.
Cell type AFC PEMFC DMFC PAFC MCFC SOFC
Proton- Proton- Li 2CO 3;
KOH conducting conducting Phosphoric KCO 3 ZrO 2 and
Electrolyte
Solution polymer polymer acid melted in Y 2O 3
membrane membrane LiALO 2
Ions in the OH – H + H + H + 2– O
2–
electrolyte CO 3
600– 700–
Temperature 60–80°°C 60–100°C 60–100°C 180–220°C
660°C 1,000°C
H 2 (pure
H 2 (pure or H 2 (pure or H 2 (pure or
Fuel H 2 Methanol or
reformed) reformed) reformed)
reformed)
Automobile,
Cogeneration
Application Spatial portable, Portable Cogeneration centralized electricity
domains cogeneration production, maritime
and maritime
Table 1.2. Comparison of fuel cells