Page 53 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 53
42 Introduction to Transfer Phenomena in PEM Fuel Cells
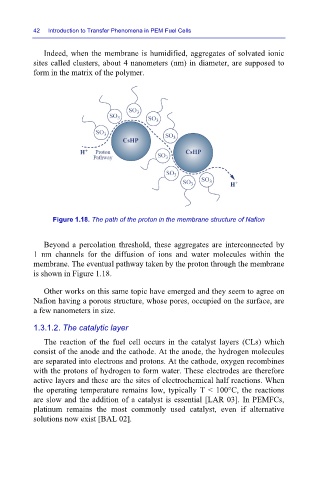
Indeed, when the membrane is humidified, aggregates of solvated ionic
sites called clusters, about 4 nanometers (nm) in diameter, are supposed to
form in the matrix of the polymer.
Figure 1.18. The path of the proton in the membrane structure of Nafion
Beyond a percolation threshold, these aggregates are interconnected by
1 nm channels for the diffusion of ions and water molecules within the
membrane. The eventual pathway taken by the proton through the membrane
is shown in Figure 1.18.
Other works on this same topic have emerged and they seem to agree on
Nafion having a porous structure, whose pores, occupied on the surface, are
a few nanometers in size.
1.3.1.2. The catalytic layer
The reaction of the fuel cell occurs in the catalyst layers (CLs) which
consist of the anode and the cathode. At the anode, the hydrogen molecules
are separated into electrons and protons. At the cathode, oxygen recombines
with the protons of hydrogen to form water. These electrodes are therefore
active layers and these are the sites of electrochemical half reactions. When
the operating temperature remains low, typically T < 100°C, the reactions
are slow and the addition of a catalyst is essential [LAR 03]. In PEMFCs,
platinum remains the most commonly used catalyst, even if alternative
solutions now exist [BAL 02].