Page 125 - Introduction to chemical reaction engineering and kinetics
P. 125
5.6 Complexities Combined 107
r
0.2 0.4 0.6 0.8 1
Conversion (f)
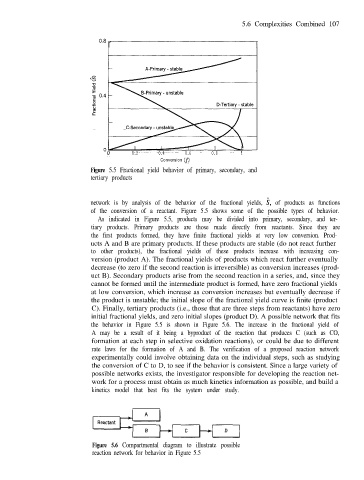
Figure 5.5 Fractional yield behavior of primary, secondary, and
tertiary products
network is by analysis of the behavior of the fractional yields, i, of products as functions
of the conversion of a reactant. Figure 5.5 shows some of the possible types of behavior.
As indicated in Figure 5.5, products may be divided into primary, secondary, and ter-
tiary products. Primary products are those made directly from reactants. Since they are
the first products formed, they have finite fractional yields at very low conversion. Prod-
ucts A and B are primary products. If these products are stable (do not react further
to other products), the fractional yields of these products increase with increasing con-
version (product A). The fractional yields of products which react further eventually
decrease (to zero if the second reaction is irreversible) as conversion increases (prod-
uct B). Secondary products arise from the second reaction in a series, and, since they
cannot be formed until the intermediate product is formed, have zero fractional yields
at low conversion, which increase as conversion increases but eventually decrease if
the product is unstable; the initial slope of the fractional yield curve is finite (product
C). Finally, tertiary products (i.e., those that are three steps from reactants) have zero
initial fractional yields, and zero initial slopes (product D). A possible network that fits
the behavior in Figure 5.5 is shown in Figure 5.6. The increase in the fractional yield of
A may be a result of it being a byproduct of the reaction that produces C (such as CO,
formation at each step in selective oxidation reactions), or could be due to different
rate laws for the formation of A and B. The verification of a proposed reaction network
experimentally could involve obtaining data on the individual steps, such as studying
the conversion of C to D, to see if the behavior is consistent. Since a large variety of
possible networks exists, the investigator responsible for developing the reaction net-
work for a process must obtain as much kinetics information as possible, and build a
kinetics model that best fits the system under study.
Figure 5.6 Compartmental diagram to illustrate possible
reaction network for behavior in Figure 5.5