Page 121 - Introduction to chemical reaction engineering and kinetics
P. 121
5.5 Series Reactions 103
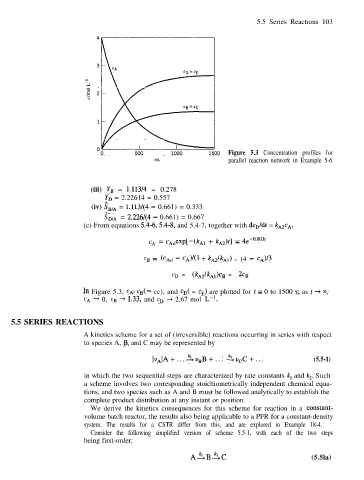
Figure 5.3 Concentration profiles for
tls parallel reaction network in Example 5-6
(iii) Yu = 1.113/4 = 0.278
y6 = 2.22614 = 0.557
(iv) SAn,* = 1.113/(4 - 0.661) = 0.333
SD,A = 2.226/(4 - 0.661) = 0.667
(c) From equations 5.4-6,5.4-8, and 5.4-7, together with dcnldt = kA2cA,
CA = C,&eXp[-(kAl + k&t] = 4e-0’003t
cl3 = (CA0 - cA>/(l + k,&kAl) = (4 - CA)/3
CD = (k,,/k,,)C, = 2c,
In Figure 5.3, CA, cn(= cc), and cn( = cn) are plotted for t = 0 to 1500 s; as t + co,
CA + 0, cB -+ 1.33, and cn + 2.67 mol L-l.
5.5 SERIES REACTIONS
A kinetics scheme for a set of (irreversible) reactions occurring in series with respect
to species A, B, and C may be represented by
lvAIA+...k’-vnB+... %I@+... (5.5-1)
in which the two sequential steps are characterized by rate constants k, and k,. Such
a scheme involves two corresponding stoichiometrically independent chemical equa-
tions, and two species such as A and B must be followed analytically to establish the
complete product distribution at any instant or position.
We derive the kinetics consequences for this scheme for reaction in a constant-
volume batch reactor, the results also being applicable to a PFR for a constant-density
system. The results for a CSTR differ from this, and are explored in Example 18-4.
Consider the following simplified version of scheme 5.5-1, with each of the two steps
being first-order:
A.%B&C (5.5la)