Page 119 - Introduction to chemical reaction engineering and kinetics
P. 119
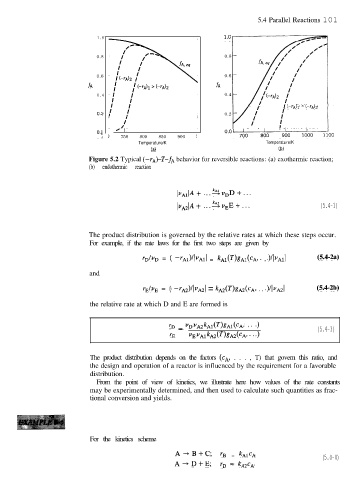
5.4 Parallel Reactions 101
1 . 0
0.8 0.8
0.6 0.6
fA fA
0 . 4 0.4
/ (-r,j)l (-42
0.2 0.2
I I I
0.’ I 750 800 850 900 !
Temperature/K Temperature/K
(a) (b)
Figure 5.2 Typical (-rA)-T-fA behavior for reversible reactions: (a) exothermic reaction;
(b) endothermic reaction
IVAllA + . ..%vpD+...
I1.“4*lA + . ..%v.E+... (5.4-1)
The product distribution is governed by the relative rates at which these steps occur.
For example, if the rate laws for the first two steps are given by
rDIvD = ( -c41)4v*1l = k*1(%41(C‘4~~ . .YlY4Il (5.4-2a)
and
IEl.V,E = ( -%2Y1?421 = kd%42k4~ * * MY421 (5.4-2b)
the relative rate at which D and E are formed is
rD _ vDVA2kAl(TkA1(CA~ * * *>
- - (5.4-3)
rE ~Ev*lkA2(~)g‘42(CA~~ *.>
The product distribution depends on the factors (cA, . . . , T) that govern this ratio, and
the design and operation of a reactor is influenced by the requirement for a favorable
distribution.
From the point of view of kinetics, we illustrate here how values of the rate constants
may be experimentally determined, and then used to calculate such quantities as frac-
tional conversion and yields.
For the kinetics scheme
A+B+C; rB = kAICA (5.4-4)
A+D+E; rD = lCAZCA