Page 197 - Introduction to chemical reaction engineering and kinetics
P. 197
8.1 Catalysis and Catalysts 179
reaction rates (this is developed in Section 8.5). Approximately 80% of commer-
cial catalytic reactions involve heterogeneous catalysis. This is due to the gener-
ally greater flexibility compared with homogeneous catalysis, and to the added
cost of separation of the catalyst from a homogeneous system.
8.1.3 General Aspects of Catalysis
8.1.3.1 Catalytic Sites
Central to catalysis is the notion of the catalytic “site.” It is defined as the catalytic
center involved in the reaction steps, and, in Figure 8.1, is the molybdenum atom
where the reactions take place. Since all catalytic centers are the same for molec-
ular catalysts, the elementary steps are bimolecular or unimolecular steps with the
same rate laws which characterize the homogeneous reactions in Chapter 7. How-
ever, if the reaction takes place in solution, the individual rate constants may de-
pend on the nonreactive ligands and the solution composition in addition to tempera-
ture.
For catalytic reactions which take place on surfaces, the term “catalytic site” is used
to describe a location on the surface which bonds with reaction intermediates. This
involves a somewhat arbitrary division of the continuous surface into smaller ensembles
of atoms. This and other points about surface catalysts can be discussed by reference
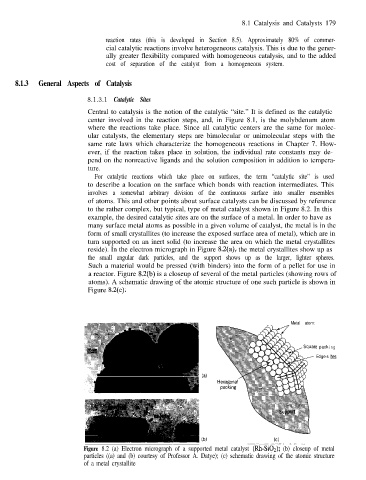
to the rather complex, but typical, type of metal catalyst shown in Figure 8.2. In this
example, the desired catalytic sites are on the surface of a metal. In order to have as
many surface metal atoms as possible in a given volume of catalyst, the metal is in the
form of small crystallites (to increase the exposed surface area of metal), which are in
turn supported on an inert solid (to increase the area on which the metal crystallites
reside). In the electron micrograph in Figure 8.2(a), the metal crystallites show up as
the small angular dark particles, and the support shows up as the larger, lighter spheres.
Such a material would be pressed (with binders) into the form of a pellet for use in
a reactor. Figure 8.2(b) is a closeup of several of the metal particles (showing rows of
atoms). A schematic drawing of the atomic structure of one such particle is shown in
Figure 8.2(c).
Metal atom:
-e pack i n g
Edge s ,ites
(a)
(b)
Figure 8.2 (a) Electron micrograph of a supported metal catalyst (Rh-SiO,); (b) closeup of metal
particles ((a) and (b) courtesy of Professor A. Datye); (c) schematic drawing of the atomic structure
of a metal crystallite