Page 202 - Introduction to chemical reaction engineering and kinetics
P. 202
184 Chapter 8: Catalysis and Catalytic Reactions
where k, is the rate constant at sufficiently low concentrations of both H+ and OH- (as,
perhaps, in a neutral solution at pH = 7) kH+ is the hydrogen-ion catalytic rate constant,
and koH- is the hydroxyl-ion catalytic rate constant. If only the kH+ cu+ term is impor-
tant, we have specific hydrogen-ion catalysis, and correspondingly for the koH-cOH-
term. Since the ion-product constant of water, K,, is
K, = CH+COHm (8.2-4)
equation 8.2-3 may be written as
k ohs = k, + kH+ CH+ + ko,- K,,,lcH+ (8.2-5)
where the value of K, is 1.0 X lo-t4 mo12 LP2 at 25°C.
If only one term in equation 8.2-3 or 8.2-5 predominates in a particular region of
pH, various cases can arise, and these may be characterized or detected most readily if
equation 8.2-5 is put into logarithmic form:
lo&O kobs = (constant) t loglo cH+ (8.2-6)
= (constant) ? pH (8.2-6a)
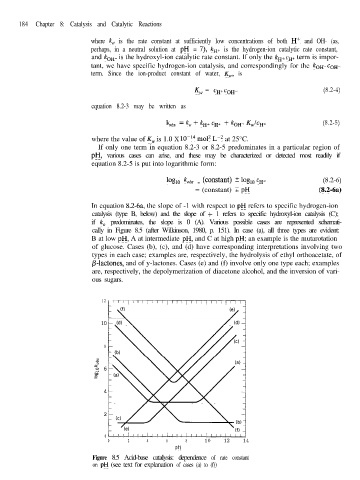
In equation 8.2-6a, the slope of -1 with respect to pH refers to specific hydrogen-ion
catalysis (type B, below) and the slope of + 1 refers to specific hydroxyl-ion catalysis (C);
if k, predominates, the slope is 0 (A). Various possible cases are represented schemati-
cally in Figure 8.5 (after Wilkinson, 1980, p. 151). In case (a), all three types are evident:
B at low pH, A at intermediate pH, and C at high pH; an example is the mutarotation
of glucose. Cases (b), (c), and (d) have corresponding interpretations involving two
types in each case; examples are, respectively, the hydrolysis of ethyl orthoacetate, of
p-lactones, and of y-lactones. Cases (e) and (f) involve only one type each; examples
are, respectively, the depolymerization of diacetone alcohol, and the inversion of vari-
ous sugars.
12
8
0
0 2 4 6 8 10 12 14
PH
Figure 8.5 Acid-base catalysis: dependence of rate constant
on pH (see text for explanation of cases (a) to (f))