Page 207 - Introduction to chemical reaction engineering and kinetics
P. 207
8.3 Autocatalysis 189
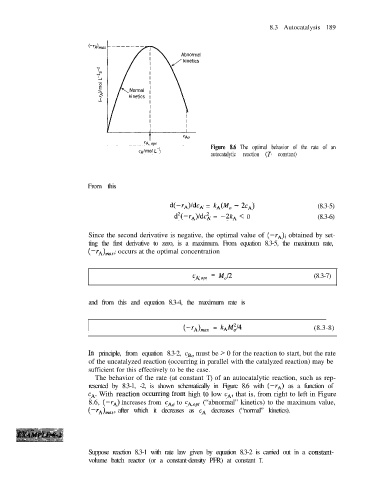
Figure 8.6 The optimal behavior of the rate of an
CA/m01 L-l
autocatalytic reaction (T constant)
From this
d(-rA)ldc, = k,(M, - 2cA) (8.3-5)
d’(-q$dc: = -2k, < 0 (8.3-6)
Since the second derivative is negative, the optimal value of (--I*), obtained by set-
ting the first derivative to zero, is a maximum. From equation 8.3-5, the maximum rate,
(-r*Lax~ occurs at the optimal concentration
CA, opt = M,/2 (8.3-7)
and from this and equation 8.3-4, the maximum rate is
(-rA)mx = kAM$ (8.3-8) )
In principle, from equation 8.3-2, cBO must be > 0 for the reaction to start, but the rate
of the uncatalyzed reaction (occurring in parallel with the catalyzed reaction) may be
sufficient for this effectively to be the case.
The behavior of the rate (at constant T) of an autocatalytic reaction, such as rep-
resented by 8.3-1, -2, is shown schematically in Figure 8.6 with (-rA) as a function of
CA. With reaction OCCurring from high t0 low CA, that is, from right to left in Figure
8.6, (-rA) increases from CA0 to cA,opt (“abnormal” kinetics) to the maximum value,
after which it decreases as CA decreases (“normal” kinetics).
(-rAhnax,
Suppose reaction 8.3-1 with rate law given by equation 8.3-2 is carried out in a constant-
volume batch reactor (or a constant-density PFR) at constant T.