Page 211 - Introduction to chemical reaction engineering and kinetics
P. 211
8.4 Surface Catalysis: Intrinsic Kinetics 193
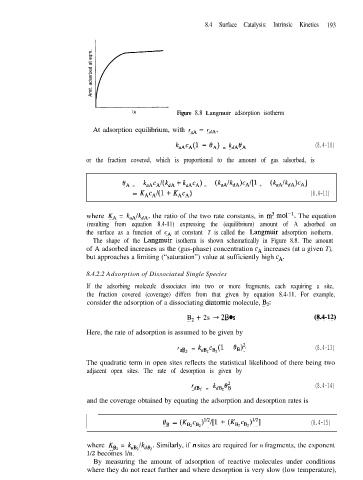
CA Figure 8.8 Langmuir adsorption isotherm
At adsorption equilibrium, with r,A = r&,
(8.4-10)
‘~IA~A(~ - eA> = kdAeA
or the fraction covered, which is proportional to the amount of gas adsorbed, is
eA = kaACAi(kdA + kzACA) = (‘%AikdAkA@ + hzAikdAkAl
= ZCAcAl(l + KAcA) (8.4-11)
where KA = kaA/kdA, the ratio of the two rate constants, in m3 mol-I. The equation
(resulting from equation 8.4-11) expressing the (equilibrium) amount of A adsorbed on
the surface as a function of CA at constant T is called the Langmuir adsorption isotherm.
The shape of the Langmuir isotherm is shown schematically in Figure 8.8. The amount
of A adsorbed increases as the (gas-phase) concentration cA increases (at a given T),
but approaches a limiting (“saturation”) value at sufficiently high CA.
8.4.2.2 Adsorption of Dissociated Single Species
If the adsorbing molecule dissociates into two or more fragments, each requiring a site,
the fraction covered (coverage) differs from that given by equation 8.4-11. For example,
consider the adsorption of a dissociating diatomic molecule, B,:
B, + 2s + 2B.s (8.4-12)
Here, the rate of adsorption is assumed to be given by
r a&? = katg& - hd* (8.4-13)
The quadratic term in open sites reflects the statistical likelihood of there being two
adjacent open sites. The rate of desorption is given by
(8.4-14)
rdEz = kdB2f%
and the coverage obtained by equating the adsorption and desorption rates is
eB = (KQ%$‘~/[~ + (KB,cB,)1’21 (8.4-15) /
where KB, = kaBz/kdB,. Similarly, if n sites are required for n fragments, the exponent
1/2 becomes l/n.
By measuring the amount of adsorption of reactive molecules under conditions
where they do not react further and where desorption is very slow (low temperature),