Page 209 - Introduction to chemical reaction engineering and kinetics
P. 209
8.4 Surface Catalysis: Intrinsic Kinetics 191
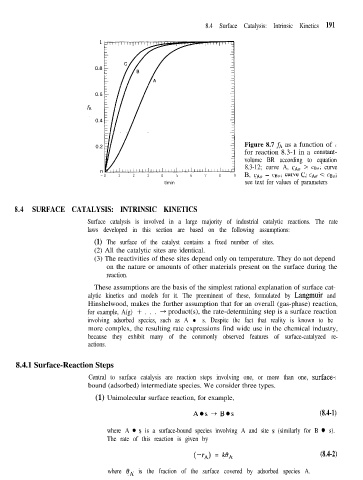
Figure 8.7 f~ as a function of t
for reaction 8.3-1 in a constant-
volume BR according to equation
8.3-12; curve A, CA0 > cBO; curve
-0 1 2 3 4 5 6 7 8 9 B, CA0 = c&; curve c, CA0 < cB&
tlmin see text for values of parameters
8.4 SURFACE CATALYSIS: INTRINSIC KINETICS
Surface catalysis is involved in a large majority of industrial catalytic reactions. The rate
laws developed in this section are based on the following assumptions:
(1) The surface of the catalyst contains a fixed number of sites.
(2) All the catalytic sites are identical.
(3) The reactivities of these sites depend only on temperature. They do not depend
on the nature or amounts of other materials present on the surface during the
reaction.
These assumptions are the basis of the simplest rational explanation of surface cat-
alytic kinetics and models for it. The preeminent of these, formulated by Langmuir and
Hinshelwood, makes the further assumption that for an overall (gas-phase) reaction,
for example, A(g) + . . . + product(s), the rate-determining step is a surface reaction
involving adsorbed species, such as A l s. Despite the fact that reality is known to be
more complex, the resulting rate expressions find wide use in the chemical industry,
because they exhibit many of the commonly observed features of surface-catalyzed re-
actions.
8.4.1 Surface-Reaction Steps
Central to surface catalysis are reaction steps involving one, or more than one, surface-
bound (adsorbed) intermediate species. We consider three types.
(1) Unimolecular surface reaction, for example,
A.s -+ B.s (8.4-1)
where A 0 s is a surface-bound species involving A and site s (similarly for B 0 s).
The rate of this reaction is given by
(-r-J = k6, (8.4-2)
where 0, is the fraction of the surface covered by adsorbed species A.