Page 259 - Introduction to chemical reaction engineering and kinetics
P. 259
240 Chapter 9: Multiphase Reacting Systems
9.2.2 Two-Film Mass-Transfer Model for Gas-Liquid Systems
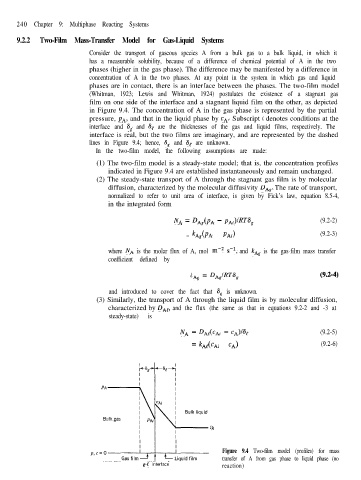
Consider the transport of gaseous species A from a bulk gas to a bulk liquid, in which it
has a measurable solubility, because of a difference of chemical potential of A in the two
phases (higher in the gas phase). The difference may be manifested by a difference in
concentration of A in the two phases. At any point in the system in which gas and liquid
phases are in contact, there is an interface between the phases. The two-film model
(Whitman, 1923; Lewis and Whitman, 1924) postulates the existence of a stagnant gas
film on one side of the interface and a stagnant liquid film on the other, as depicted
in Figure 9.4. The concentration of A in the gas phase is represented by the partial
pressure, PA, and that in the liquid phase by cA. Subscript i denotes conditions at the
interface and 6, and 8, are the thicknesses of the gas and liquid films, respectively. The
interface is real, but the two films are imaginary, and are represented by the dashed
lines in Figure 9.4; hence, 6, and 6, are unknown.
In the two-film model, the following assumptions are made:
(1) The two-film model is a steady-state model; that is, the concentration profiles
indicated in Figure 9.4 are established instantaneously and remain unchanged.
(2) The steady-state transport of A through the stagnant gas film is by molecular
diffusion, characterized by the molecular diffusivity DA,. The rate of transport,
normalized to refer to unit area of interface, is given by Fick’s law, equation 8.5-4,
in the integrated form
(9.2-2)
NA = D~g(PA - pAi)IRTSg
= kAg(PA - PAi) (9.2-3)
where NA is the molar flux of A, mol mP2 s-l, and kAg is the gas-film mass transfer
coefficient defined by
k Ag = DAJRTS,
and introduced to cover the fact that S, is unknown.
(3) Similarly, the transport of A through the liquid film is by molecular diffusion,
characterized by DA,, and the flux (the same as that in equations 9.2-2 and -3 at
steady-state) is
NA = DAdCAi - CA)lBb (9.2-5)
(9.2-6)
= kAt(CAi - cA)
CA
Figure 9.4 Two-film model (profiles) for mass
transfer of A from gas phase to liquid phase (no
g-e interface reaction)