Page 263 - Introduction to chemical reaction engineering and kinetics
P. 263
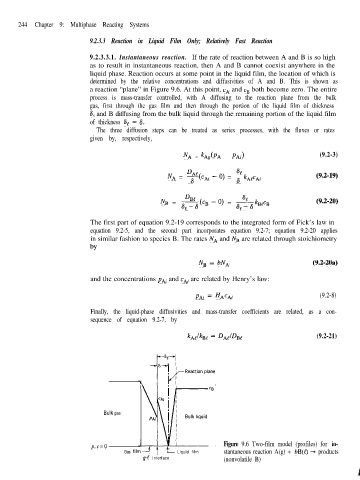
244 Chapter 9: Multiphase Reacting Systems
9.2.3.3 Reaction in Liquid Film Only; Relatively Fast Reaction
9.2.3.3.1. Instantaneous reaction. If the rate of reaction between A and B is so high
as to result in instantaneous reaction, then A and B cannot coexist anywhere in the
liquid phase. Reaction occurs at some point in the liquid film, the location of which is
determined by the relative concentrations and diffusivities of A and B. This is shown as
a reaction “plane” in Figure 9.6. At this point, cA and cn both become zero. The entire
process is mass-transfer controlled, with A diffusing to the reaction plane from the bulk
gas, first through the gas film and then through the portion of the liquid film of thickness
6, and B diffusing from the bulk liquid through the remaining portion of the liquid film
of thickness 6, - S.
The three diffusion steps can be treated as series processes, with the fluxes or rates
given by, respectively,
(9.2-3)
NA = kAg(PA - PAi)
se
NA = %(c,i - 0) = s kAecAi
&I = &(c, - 0) = -f-&k&B
The first part of equation 9.2-19 corresponds to the integrated form of Fick’s law in
equation 9.2-5, and the second part incorporates equation 9.2-7; equation 9.2-20 applies
in similar fashion to species B. The rates NA and Nn are related through stoichiometry
bY
NB = bNA (9.2-20a)
and the concentrations PAi and cAi are related by Henry’s law:
PAi = HACAi (9.2-8)
Finally, the liquid-phase diffusivities and mass-transfer coefficients are related, as a con-
sequence of equation 9.2-7, by
(9.2-21)
3
Bulk gas
p,c=o Figure 9.6 Two-film model (profiles) for in-
Gas film2 C Liquid film stantaneous reaction A(g) + bB(t) + products
g-e interface (nonvolatile B)