Page 266 - Introduction to chemical reaction engineering and kinetics
P. 266
9.2 Gas-Liquid Systems 247
since cA = 0 for maximum rate of mass transfer. Thus, in terms of E, the rate law is
(-‘A) = kAfEcAi (9.2-26a) )
The interfacial concentration cAi can be eliminated by means of equations 9.2-3 and -8
to give
(-rA) = ‘A~* (9.2-27)
‘+-
k*S kA&
For an instantaneous reaction, equation 9.2-27 is an alternative to equation 9.2-22. An
expression for E can be obtained from these two equations, and special cases can be
examined in terms of E (see problems 9-11 and 9-12).
9.2.3.4 Reaction in Liquid Film and Bulk Liquid
The cases above, reaction in bulk liquid only and instantaneous reaction in the liquid
film, have been treated by considering rate processes in series. We can’t use this ap-
proach if diffusion and reaction of A and B are both spread over the liquid film. Instead,
we consider solution of the continuity equations for A and B, through the liquid film.
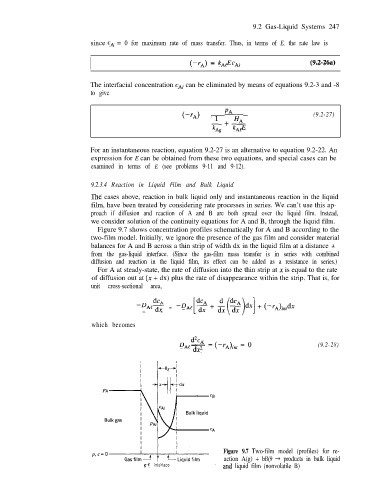
Figure 9.7 shows concentration profiles schematically for A and B according to the
two-film model. Initially, we ignore the presence of the gas film and consider material
balances for A and B across a thin strip of width dx in the liquid film at a distance x
from the gas-liquid interface. (Since the gas-film mass transfer is in series with combined
diffusion and reaction in the liquid film, its effect can be added as a resistance in series.)
For A at steady-state, the rate of diffusion into the thin strip at x is equal to the rate
of diffusion out at (x + dx) plus the rate of disappearance within the strip. That is, for
unit cross-sectional area,
dCA
-DAfx = -DA, [z + &($$)dX] + (-rA)intdX
which becomes
d2CA = 0 (9.2-28)
- - (-?-A)int
DAe dx2
Figure 9.7 Two-film model (profiles) for re-
action A(g) + bB(9 + products in bulk liquid
g-l interface and liquid film (nonvolatile B)