Page 284 - Introduction to chemical reaction engineering and kinetics
P. 284
10.2 Models of Enzyme Kinetics 265
Combining equation 10.2-4 with 10.2-3 and the definition of Km, we obtain
kr%oCs (10.24)
‘-’ = K,,, + cs
The form of the equation 10.2-5 is consistent with the experimental observations
(Section 10.1.2) about the dependence of rate in general on cEO, and about the de-
pendence of the initial rate on cs. Thus, r, [or (-rs)] m CEO, and the initial rate,
given by
(10.2-6)
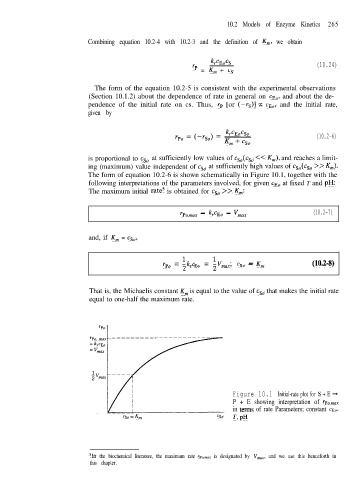
is proportional to csO at sufficiently low values of csO(csO << Km), and reaches a limit-
ing (maximum) value independent of csO at sufficiently high values of csO(csO >> Km).
The form of equation 10.2-6 is shown schematically in Figure 10.1, together with the
following interpretations of the parameters involved, for given CEO at fixed T and pH:
The maximum initial rate’ is obtained for csO >> K,,,:
~Po,max = kr% = vmax (10.2-7)
and, if K,,, = cso,
Ike r
% = 2 Eo = maxi cSo -- Km (10.2-8)
That is, the Michaelis constant K,,, is equal to the value of csO that makes the initial rate
equal to one-half the maximum rate.
Figure 10.1 Initial-rate plot for S E +
+
P + E showing interpretation of rp,,,,
in terns of rate Parameters; constant CEO,
cso = Km 30 T,pH
51n the biochemical literature, the maximum rate rPPo,man is designated by Vm,, and we use this henceforth in
this chapter.