Page 79 - Introduction to chemical reaction engineering and kinetics
P. 79
3.6 Problems for Chapter 3 61
Type of
reactor
I
Is an analytical solution
linear with respect linear with respect of the differential
to the parameters? to the parameters? equation available?
Linear Nonlinear
regression regression
eqn. 2.4-4 eqn. 2.4-4
with with
eqn. 3.4-18 eqn. 3.4-17
r-l
Is the equation
linear with respect
to the parameters?
Nonlinear
regression
3.4-9
eqn. 3.4-10
eqn.
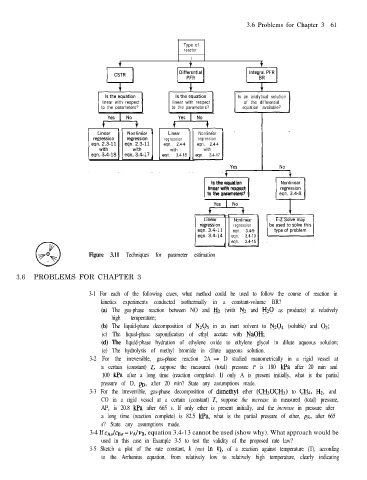
0 Figure 3.11 Techniques for parameter estimation eqn. 3.4-15
v
7O-v
3.6 PROBLEMS FOR CHAPTER 3
3-1 For each of the following cases, what method could be used to follow the course of reaction in
kinetics experiments conducted isothermally in a constant-volume BR?
(a) The gas-phase reaction between NO and Hz (with N2 and Hz0 as products) at relatively
high temperature;
(b) The liquid-phase decomposition of N205 in an inert solvent to N204 (soluble) and 02;
(c) The liquid-phase saponification of ethyl acetate with NaOH;
(d) The liquid-phase hydration of ethylene oxide to ethylene glycol in dilute aqueous solution;
(e) The hydrolysis of methyl bromide in dilute aqueous solution.
3-2 For the irreversible, gas-phase reaction 2A + D studied manometrically in a rigid vessel at
a certain (constant) T, suppose the measured (total) pressure P is 180 kPa after 20 min and
100 kPa after a long time (reaction complete). If only A is present initially, what is the partial
pressure of D, po, after 20 min? State any assumptions made.
3-3 For the irreversible, gas-phase decomposition of dimethyl ether (CH30CH3) to GIL Hz, and
CO in a rigid vessel at a certain (constant) T, suppose the increase in measured (total) pressure,
AP, is 20.8 kPa after 665 s. If only ether is present initially, and the increase in pressure after
a long time (reaction complete) is 82.5 kPa, what is the partial pressure of ether, PE, after 665
s? State any assumptions made.
3-4 If c.&cnO = v~Ivn, equation 3.4-13 cannot be used (show why). What approach would be
used in this case in Example 3-5 to test the validity of the proposed rate law?
3-5 Sketch a plot of the rate constant, k (not In k), of a reaction against temperature (T), according
to the Arrhenius equation, from relatively low to relatively high temperature, clearly indicating