Page 78 - Introduction to chemical reaction engineering and kinetics
P. 78
60 Chapter 3: Experimental Methods in Kinetics: Measurement of Rate of Reaction
- 1st order
- - - 2nd order
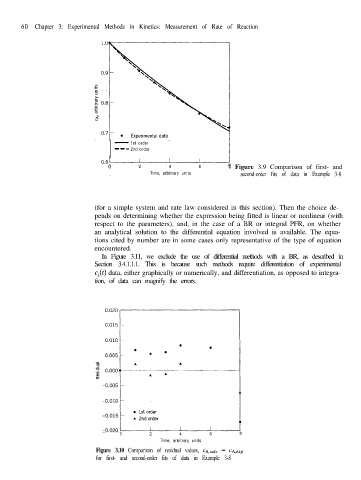
2 4 6 8 i Figure 3.9 Comparison of first- and
Time, arbitrary units second-order fits of data in Example 3-8
(for a simple system and rate law considered in this section). Then the choice de-
pends on determining whether the expression being fitted is linear or nonlinear (with
respect to the parameters), and, in the case of a BR or integral PFR, on whether
an analytical solution to the differential equation involved is available. The equa-
tions cited by number are in some cases only representative of the type of equation
encountered.
In Figure 3.11, we exclude the use of differential methods with a BR, as described in
Section 3.4.1.1.1. This is because such methods require differentiation of experimental
ci(t) data, either graphically or numerically, and differentiation, as opposed to integra-
tion, of data can magnify the errors.
O 2 4 6 8
Time, arbitrary units
Figure 3.10 Comparison of residual values, CA&c - cA,eIP
for first- and second-order fits of data in Example 3-8