Page 89 - Introduction to chemical reaction engineering and kinetics
P. 89
4.3 Dependence of Rate on Concentration 71
1.8
A
1.6
0.8
0.6
0 5 0 100 150 200 250 300 350 400
tlmin
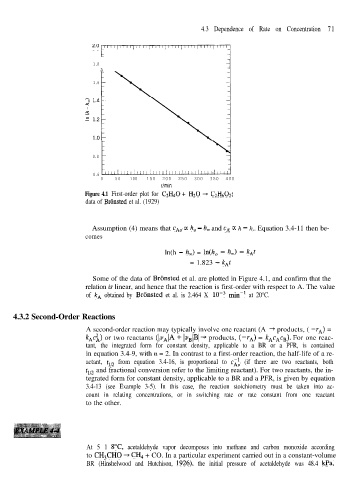
Figure 4.1 First-order plot for CzH40 + Hz0 + C2H602;
data of Briinsted et al. (1929)
Assumption (4) means that cAO 0~ h, - h, and cA K h - h,. Equation 3.4-11 then be-
comes
ln(h - h,) = ln(h, - h,) - Kit
= 1.823 - kAt
Some of the data of Bronsted et al. are plotted in Figure 4.1, and confirm that the
relation is linear, and hence that the reaction is first-order with respect to A. The value
of kA obtained by Brijnsted et al. is 2.464 X lop3 mini at 20°C.
4.3.2 Second-Order Reactions
A second-order reaction may typically involve one reactant (A + products, ( -rA) =
k,c$J or two reactants (Iv*IA + Iv,lB + products, (-I*) = kAcAcB). For one reac-
tant, the integrated form for constant density, applicable to a BR or a PFR, is contained
in equation 3.4-9, with n = 2. In contrast to a first-order reaction, the half-life of a re-
actant, t1,2 from equation 3.4-16, is proportional to CA: (if there are two reactants, both
t1,2 and fractional conversion refer to the limiting reactant). For two reactants, the in-
tegrated form for constant density, applicable to a BR and a PFR, is given by equation
3.4-13 (see Example 3-5). In this case, the reaction stoichiometry must be taken into ac-
count in relating concentrations, or in switching rate or rate constant from one reactant
to the other.
At 5 1 VC, acetaldehyde vapor decomposes into methane and carbon monoxide according
to CHsCHO + CH, + CO. In a particular experiment carried out in a constant-volume
BR (Hinshelwood and Hutchison, 1926), the initial pressure of acetaldehyde was 48.4 kPa,