Page 191 - Materials Chemistry, Second Edition
P. 191
Sustainability of (H 2 ? CH 4 ) by Anaerobic Digestion 179
production of two high-value gases, such as hydrogen and methane, is a solution
which leads to several energy and environmental advantages: two separate fluxes
of high-energy value gas (H 2 and CH 4 ), optimization of the AD process for the
treatment of refuse and its control (Monnet 2003). The produced biogas (CH 4 and/
or H 2 ) can be used to create a source of income: biogas can be upgraded removing
carbon dioxide and water vapor, and then, for example, used in a cogeneration unit
as combined heat and power (CHP) to produce electricity and heat. The digestate
either liquid or solid can instead be used as a fertilizer, or further processed into
compost or high-value products, as bioproducts, e.g., acetic and butyric acids
(Angenent et al. 2004).
3.1.1 Hydrogen and Methane Production in Two-Steps AD
Anaerobic digestion, from a biological point of view, is a multistep process that
involves the action of various microbial species (Lyberatos and Skiadas 1999).
Usually, such a process contains a particular step, the so-called rate-limiting step,
which, being the slowest, limits the rate of the overall process (Hill 1977).
However, the limiting step is not always the same over a wide range of operating
conditions. It depends on the waste characteristics, hydraulic retention time,
temperature, and many others (Speece 1983). The two-steps AD process is a
process in which hydrogen and methane are produced in two separate bioreactors
through the separation of hydrogen forming bacteria from methane forming bac-
teria (Tommasi 2011; Gómez et al. 2011) working in different conditions such as
pH and hydraulic retention time. This partition, optimizing the fermentation pro-
cess, permits the production of two high-value gases by splitting acetogenesis from
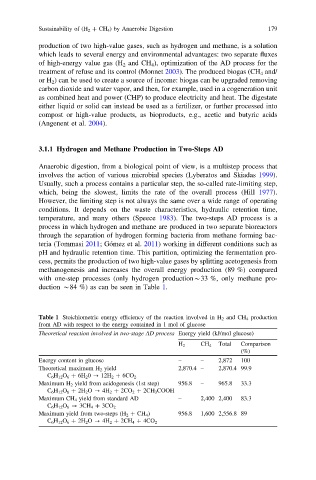
methanogenesis and increases the overall energy production (89 %) compared
with one-step processes (only hydrogen production*33 %, only methane pro-
duction *84 %) as can be seen in Table 1.
Table 1 Stoichiometric energy efficiency of the reaction involved in H 2 and CH 4 production
from AD with respect to the energy contained in 1 mol of glucose
Theoretical reaction involved in two-stage AD process Energy yield (kJ/mol glucose)
H 2 CH 4 Total Comparison
(%)
Energy content in glucose – – 2,872 100
Theoretical maximum H 2 yield 2,870.4 – 2,870.4 99.9
C 6 H 12 O 6 ? 6H 2 0 ? 12H 2 ? 6CO 2
Maximum H 2 yield from acidogenesis (1st step) 956.8 – 965.8 33.3
C 6 H 12 O 6 ? 2H 2 O ? 4H 2 ? 2CO 2 ? 2CH 3 COOH
Maximum CH 4 yield from standard AD – 2,400 2,400 83.3
C 6 H 12 O 6 ? 3CH 4 ? 3CO 2
Maximum yield from two-steps (H 2 ? CH 4 ) 956.8 1,600 2,556.8 89
C 6 H 12 O 6 ? 2H 2 O ? 4H 2 ? 2CH 4 ? 4CO 2