Page 267 - Lignocellulosic Biomass to Liquid Biofuels
P. 267
Fischer Tropsch synthesis of syngas to liquid hydrocarbons 223
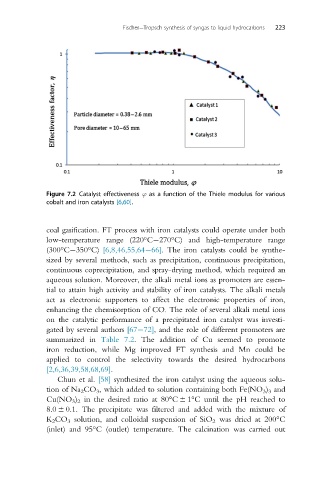
Figure 7.2 Catalyst effectiveness ϕ as a function of the Thiele modulus for various
cobalt and iron catalysts [6,60].
coal gasification. FT process with iron catalysts could operate under both
low-temperature range (220°C 270°C) and high-temperature range
(300°C 350°C) [6,8,46,55,64 66]. The iron catalysts could be synthe-
sized by several methods, such as precipitation, continuous precipitation,
continuous coprecipitation, and spray-drying method, which required an
aqueous solution. Moreover, the alkali metal ions as promoters are essen-
tial to attain high activity and stability of iron catalysts. The alkali metals
act as electronic supporters to affect the electronic properties of iron,
enhancing the chemisorption of CO. The role of several alkali metal ions
on the catalytic performance of a precipitated iron catalyst was investi-
gated by several authors [67 72], and the role of different promoters are
summarized in Table 7.2. The addition of Cu seemed to promote
iron reduction, while Mg improved FT synthesis and Mn could be
applied to control the selectivity towards the desired hydrocarbons
[2,6,36,39,58,68,69].
Chun et al. [58] synthesized the iron catalyst using the aqueous solu-
tion of Na 2 CO 3 , which added to solution containing both Fe(NO 3 ) 3 and
Cu(NO 3 ) 2 in the desired ratio at 80°C 6 1°C until the pH reached to
8.0 6 0.1. The precipitate was filtered and added with the mixture of
K 2 CO 3 solution, and colloidal suspension of SiO 2 was dried at 200°C
(inlet) and 95°C (outlet) temperature. The calcination was carried out