Page 161 - Lindens Handbook of Batteries
P. 161
6.14 PRINCIPLES OF OPERATION
3.4
Measured
Fitted E s
3.2
Fitted E v
Open-circuit potential (V) 2.8
3.0
2.6
0 to 2
2.4 θ s goes from
θ goes form 0 to 4
v
2.2
0 1 2 3 4 5 6
θ + θ v
s
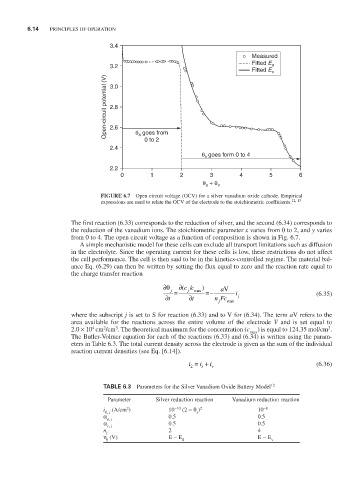
FiguRE 6.7 Open circuit voltage (OCV) for a silver vanadium oxide cathode. Empirical
expressions are used to relate the OCV of the electrode to the stoichiometric coefficients. 12, 13
The first reaction (6.33) corresponds to the reduction of silver, and the second (6.34) corresponds to
the reduction of the vanadium ions. The stoichiometric parameter x varies from 0 to 2, and y varies
from 0 to 4. The open circuit voltage as a function of composition is shown in Fig. 6.7.
A simple mechanistic model for these cells can exclude all transport limitations such as diffusion
in the electrolyte. Since the operating current for these cells is low, these restrictions do not affect
the cell performance. The cell is then said to be in the kinetics-controlled regime. The material bal-
ance Eq. (6.29) can then be written by setting the flux equal to zero and the reaction rate equal to
the charge transfer reaction
∂θ ∂ cc / ) aV
(
j = j max =- i (6.35)
j
∂t ∂t nFc
j max
where the subscript j is set to S for reaction (6.33) and to V for (6.34). The term aV refers to the
area available for the reactions across the entire volume of the electrode V and is set equal to
3
2
3
4
2.0 × 10 cm /cm . The theoretical maximum for the concentration (c max ) is equal to 124.35 mol/cm .
The Butler-Volmer equation for each of the reactions (6.33) and (6.34) is written using the param-
eters in Table 6.3. The total current density across the electrode is given as the sum of the individual
reaction current densities (see Eq. [6.14]).
i = 2 i + s i (6.36)
v
TABLE 6.3 Parameters for the Silver Vanadium Oxide Battery Model 12
Parameter Silver reduction reaction Vanadium reduction reaction
i (A/cm ) 10 -10 (2 - θ ) 2 10 -8
2
0, j
s
α a, j 0.5 0.5
α c, j 0.5 0.5
n j 2 4
η (V) E - E E - E
j S v