Page 167 - Lindens Handbook of Batteries
P. 167
6.20 PRINCIPLES OF OPERATION
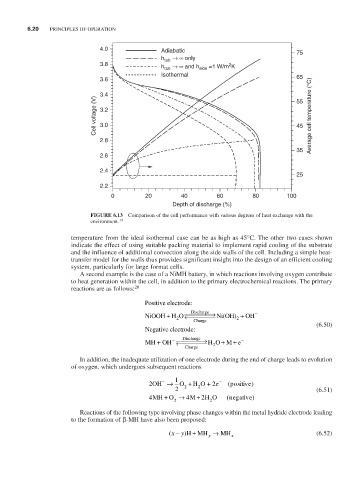
4.0 Adiabatic 75
h tab → ∞ only
3.8 2
h tab → ∞ and h side =1 W/m K
Isothermal 65
3.6
3.4 55
Cell voltage (V) 3.2 45 Average cell temperature (°C)
3.0
2.8
35
2.6
2.4
25
2.2
0 20 40 60 80 100
Depth of discharge (%)
FiguRE 6.13 Comparison of the cell performance with various degrees of heat exchange with the
19
environment.
temperature from the ideal isothermal case can be as high as 45°C. The other two cases shown
indicate the effect of using suitable packing material to implement rapid cooling of the substrate
and the influence of additional convection along the side walls of the cell. Including a simple heat-
transfer model for the walls thus provides significant insight into the design of an efficient cooling
system, particularly for large format cells.
A second example is the case of a NiMH battery, in which reactions involving oxygen contribute
to heat generation within the cell, in addition to the primary electrochemical reactions. The primary
reactions are as follows: 20
Positiveelectrode:
Discharge
→
NiOOH HO ← + 2 Ni(OH) 2 + OH -
Charge (6.50)
Negativeelectrode:
+
+
MH +OH ← - Discharge → H OM e -
2
Charge
In addition, the inadequate utilization of one electrode during the end of charge leads to evolution
of oxygen, which undergoes subsequent reactions
2OH → - 1 O + H O+ 2e - ( positive)
2 2 2 (6.51)
4MH O+ 2 → 4M + 2HO (negative)
a
2
Reactions of the following type involving phase changes within the metal hydride electrode leading
to the formation of β-MH have also been proposed:
+
(x - ) y HMH y → MH (6.52)
x