Page 168 - Lindens Handbook of Batteries
P. 168
MATHEMATICAL MODELING OF BATTERIES 6.21
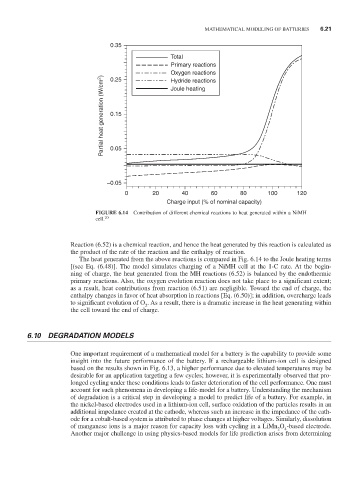
0.35
Total
Primary reactions
Oxygen reactions
Partial heat generation (W/cm 2 ) 0.15
0.25
Hydride reactions
Joule heating
0.05
–0.05
0 20 40 60 80 100 120
Charge input (% of nominal capacity)
FiguRE 6.14 Contribution of different chemical reactions to heat generated within a NiMH
cell. 20
Reaction (6.52) is a chemical reaction, and hence the heat generated by this reaction is calculated as
the product of the rate of the reaction and the enthalpy of reaction.
The heat generated from the above reactions is compared in Fig. 6.14 to the Joule heating terms
[(see Eq. (6.48)]. The model simulates charging of a NiMH cell at the 1-C rate. At the begin-
ning of charge, the heat generated from the MH reactions (6.52) is balanced by the endothermic
primary reactions. Also, the oxygen evolution reaction does not take place to a significant extent;
as a result, heat contributions from reaction (6.51) are negligible. Toward the end of charge, the
enthalpy changes in favor of heat absorption in reactions [Eq. (6.50)]; in addition, overcharge leads
to significant evolution of O . As a result, there is a dramatic increase in the heat generating within
2
the cell toward the end of charge.
6.10 DEGRADATION MODELS
One important requirement of a mathematical model for a battery is the capability to provide some
insight into the future performance of the battery. If a rechargeable lithium-ion cell is designed
based on the results shown in Fig. 6.13, a higher performance due to elevated temperatures may be
desirable for an application targeting a few cycles; however, it is experimentally observed that pro-
longed cycling under these conditions leads to faster deterioration of the cell performance. One must
account for such phenomena in developing a life-model for a battery. Understanding the mechanism
of degradation is a critical step in developing a model to predict life of a battery. For example, in
the nickel-based electrodes used in a lithium-ion cell, surface oxidation of the particles results in an
additional impedance created at the cathode, whereas such an increase in the impedance of the cath-
ode for a cobalt-based system is attributed to phase changes at higher voltages. Similarly, dissolution
of manganese ions is a major reason for capacity loss with cycling in a LiMn O -based electrode.
4
2
Another major challenge in using physics-based models for life prediction arises from determining