Page 213 - Lindens Handbook of Batteries
P. 213
ZINC-CARBON BATTERIES—LECLANCHÉ AND ZINC CHLORIDE CELL SYSTEMS 9.7
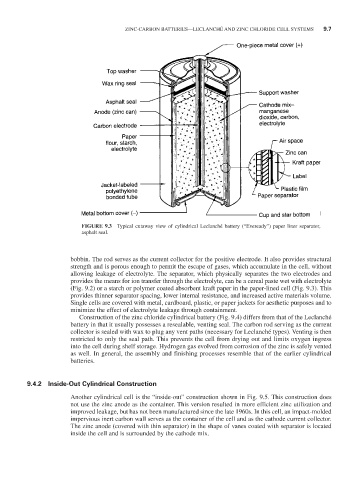
FIGURE 9.3 Typical cutaway view of cylindrical Leclanché battery (“Eveready”) paper liner separator,
asphalt seal.
bobbin. The rod serves as the current collector for the positive electrode. It also provides structural
strength and is porous enough to permit the escape of gases, which accumulate in the cell, without
allowing leakage of electrolyte. The separator, which physically separates the two electrodes and
provides the means for ion transfer through the electrolyte, can be a cereal paste wet with electrolyte
(Fig. 9.2) or a starch or polymer coated absorbent kraft paper in the paper-lined cell (Fig. 9.3). This
provides thinner separator spacing, lower internal resistance, and increased active materials volume.
Single cells are covered with metal, cardboard, plastic, or paper jackets for aesthetic purposes and to
minimize the effect of electrolyte leakage through containment.
Construction of the zinc chloride cylindrical battery (Fig. 9.4) differs from that of the Leclanché
battery in that it usually possesses a resealable, venting seal. The carbon rod serving as the current
collector is sealed with wax to plug any vent paths (necessary for Leclanché types). Venting is then
restricted to only the seal path. This prevents the cell from drying out and limits oxygen ingress
into the cell during shelf storage. Hydrogen gas evolved from corrosion of the zinc is safely vented
as well. In general, the assembly and finishing processes resemble that of the earlier cylindrical
batteries.
9.4.2 Inside-Out Cylindrical Construction
Another cylindrical cell is the “inside-out” construction shown in Fig. 9.5. This construction does
not use the zinc anode as the container. This version resulted in more efficient zinc utilization and
improved leakage, but has not been manufactured since the late 1960s. In this cell, an impact-molded
impervious inert carbon wall serves as the container of the cell and as the cathode current collector.
The zinc anode (covered with thin separator) in the shape of vanes coated with separator is located
inside the cell and is surrounded by the cathode mix.