Page 64 - Manufacturing Engineering and Technology - Kalpakjian, Serope : Schmid, Steven R.
P. 64
I I The Crystal Structure of Metals
Section 1.3
f
1-a-1 “' FC
COW
nggggg
fee
QQQJQ
5 E "s‘ :A in’ Q
\
0 |1010 J
U
°e " * l ' 1 . ¢
¢'l'|'|
.99
T
(bl
(H) l`_a1&a»| (C)
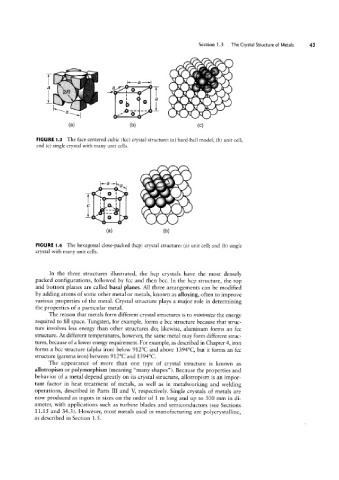
FIGURE l.3 The face-centered cubic (fcc) crystal structure: (a) hard-ball model; (b) unit cell;
and (c) single crystal with many unit cells.
C
ll
5256*
(al ef<%;¢Q*
(bl
FIGURE l.4 The hexagonal close-packed (hcp) crystal structure: (a) unit cell; and (b) single
crystal with many unit cells.
ln the three structures illustrated, the hcp crystals have the most densely
packed configurations, followed by fcc and then bcc. In the hcp structure, the top
and bottom planes are called basal planes. All three arrangements can be modified
by adding atoms of some other metal or metals, known as alloying, often to improve
various properties of the metal. Crystal structure plays a major role in determining
the properties of a particular metal.
The reason that metals form different crystal structures is to minimize the energy
required to fill space. Tungsten, for example, forms a bcc structure because that struc-
ture involves less energy than other structures do; likewise, aluminum forms an fcc
structure. At different temperatures, however, the same metal may form different struc-
tures, because of a lower energy requirement. For example, as described in Chapter 4, iron
forms a bcc structure (alpha iron) below 912°C and above 1394°C, but it forms an fcc
structure (gannna iron) between 912°C and 1394°C.
The appearance of more than one type of crystal structure is known as
allotropism or polymorphism (meaning “many shapes”). Because the properties and
behavior of a metal depend greatly on its crystal structure, allotropism is an impor-
tant factor in heat treatment of metals, as well as in metalworking and welding
operations, described in Parts Ill and \L respectively. Single crystals of metals are
now produced as ingots in sizes on the order of 1 m long and up to 300 mm in di-
ameter, with applications such as turbine blades and semiconductors (see Sections
11.15 and 34.3). However, most metals used in manufacturing are polycrystalline,
as described in Section 1.5.