Page 66 - Manufacturing Engineering and Technology - Kalpakjian, Serope : Schmid, Steven R.
P. 66
Section Deformation and Strength of Single Crystals 45
the behavior of plywood, which is much stronger in the planar Single crystal Grain
direction than along its thickness direction. Note, for example, (grain) boundaries
how plywood splits easily when a thick nail is driven through
its thickness. IQ"
The second and less common mechanism of plastic defor-
mation in crystals is twinning, in which a portion of the crystal
forms a mirror image of itself across the plane of twinning
(Fig. 1.5 b). Twins form abruptly and are the cause of the creak-
ing sound (“tin cry”) that occurs when a tin or zinc rod is bent
at room temperature. Twinning usually occurs in hcp metals. 5*
Slip Systems. The combination of a slip plane and its direc-
tion of slip is known as a slip system. In general, metals with
5 or more slip systems are ductile, whereas those with fewer
than 5 slip systems are not.
Slip lines approximately
I. In body-centered cubic crystals, there are 48 possible slip Approximately'1000i 100 atomic!
systems. Therefore, the probability is high that an exter- atomic diameters' _J/_giameters
nally applied shear stress will operate on one of these sys- Slip band(-7 /
_l_
tems and cause slip. Because of the relatively high b/ci
/
ratio in this crystal, however, the required shear stress is ai* if
high. Metals with bcc structures generally have good ~1o,ooo / fi <§§’
strength and moderate ductility, but can have high duc- ff' /' atomic f
tility at elevated temperatures. gf diameters/
2. In face-centered cubic crystals, there are 12 slip systems.
The probability of slip is moderate, and the shear stress
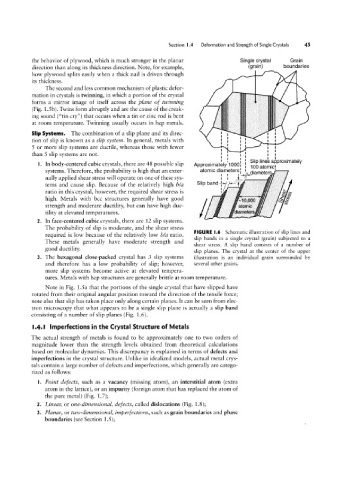
FIGURE l.6 Schematic illustration of slip lines and
required is low because of the relatively low I9/az ratio.
slip bands in a single crystal (grain) subjected to a
These metals generally have moderate strength and
shear stress. A slip band consists of a number of
good ductility.
slip planes. The crystal at the center of the upper
3. The hexagonal close-packed crystal has 3 slip systems illustration is an individual grain surrounded by
and therefore has a low probability of slip; however, several other grains.
more slip systems become active at elevated tempera-
tures. Metals with hcp structures are generally brittle at room temperature.
Note in Fig. 1.5a that the portions of the single crystal that have slipped have
rotated from their original angular position toward the direction of the tensile force;
note also that slip has taken place only along certain planes. It can be seen from elec-
tron microscopy that what appears to be a single slip plane is actually a slip band
consisting of a number of slip planes (Fig. 1.6).
l.4.I lmperfections in the Crystal Structure of Metals
The actual strength of metals is found to be approximately one to two orders of
magnitude lower than the strength levels obtained from theoretical calculations
based on molecular dynamics. This discrepancy is explained in terms of defects and
imperfections in the crystal structure. Unlike in idealized models, actual metal crys-
tals contain a large number of defects and imperfections, which generally are catego-
rized as follows:
I. Point defects, such as a vacancy (missing atom), an interstitial atom (extra
atom in the lattice), or an impurity (foreign atom that has replaced the atom of
the pure metal) (Fig. 1.7);
2. Lineai; or one-dimensional, defects, called dislocations (Fig. 1.8);
3. Planar, or tu/o-dimensional, imperfections, such as grain boundaries and phase
boundaries (see Section 1.5 );