Page 63 - 04. Subyek Engineering Materials - Manufacturing, Engineering and Technology SI 6th Edition - Serope Kalpakjian, Stephen Schmid (2009)
P. 63
Chapter 1 The Structure of Metals
by covalent bonding typically have low electrical conductivity and can have high
hardness. (Diamond, a form of covalently bonded carbon, is an example.)
° Metallic bonds. Metals have relatively few electrons in their outer orbits; thus,
they cannot complete the outer shell of other self-mated atoms. Instead, metals and
alloys form metallic bonds, whereby the available electrons are shared by all atoms
in contact. The resultant electron cloud provides attractive forces to hold the atoms
together and results in generally high thermal and electrical conductivity.
In addition to the strong attractive forces associated with electrons, weak or
secondary attractions occur between molecules. Also referred to as van der Waals
forces, these forces arise from the attraction of opposite charges without electron
transfer. As an example, water molecules consist of one oxygen and two smaller hy-
drogen atoms, located around 104° from each other. Although each molecule has a
balanced, or neutral, charge, there are more hydrogen atoms on one side of the mol-
ecule (i.e., it is a dipole), so that the molecule develops a weak attraction to nearby
oxygen atoms on that side.
l.3 The Crystal Structure of Metals
_ _ ,-
When metals solidify from a molten state, the atoms arrange themselves into various
orderly configurations, called crystals; this atomic arrangement is called crystal
structure or crystalline structure. The smallest group of atoms showing the charac-
teristic lattice structure of a particular metal is known as a unit cell. It is the building
block of a crystal, and a single crystal can have many unit cells.
The following are the three basic atomic arrangements in metals:
l. Body-centered cubic (bcc); examples: alpha iron, chromium, molybdenum,
tantalum, tungsten, and vanadium.
2. Face-centered cubic (fcc); examples: gamma iron, aluminum, copper, nickel,
lead, silver, gold, and platinum.
3. Hexagonal close-packed (hcp); examples: beryllium, cadmium, cobalt, magne-
sium, alpha titanium, zinc, and zirconium.
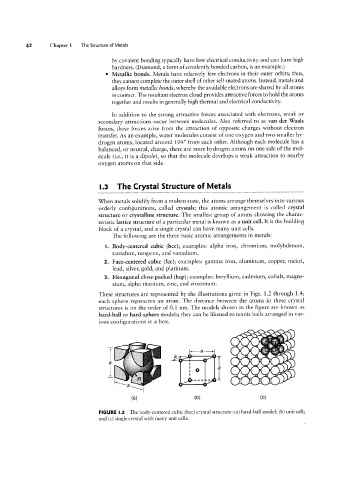
These structures are represented by the illustrations given in Figs. 1.2 through 1.4;
each sphere represents an atom. The distance between the atoms in these crystal
r "W`V§=§§§$‘
structures is on the order of 0.1 nm. The models shown in the figure are known as
hard-ball or hard-sphere models; they can be likened to tennis balls arranged in var-
ious configurations in a box.
§ § § §
.,
H r 1 °ww"»»
m. »¢~--- o»o»¢-ov’
(2) (D) (C)
FIGURE |.2 The body-centered cubic (bcc) crystal structure: (a) hard-ball model; (b) unit cell;
and (c) single crystal with many unit cells.