Page 67 - 04. Subyek Engineering Materials - Manufacturing, Engineering and Technology SI 6th Edition - Serope Kalpakjian, Stephen Schmid (2009)
P. 67
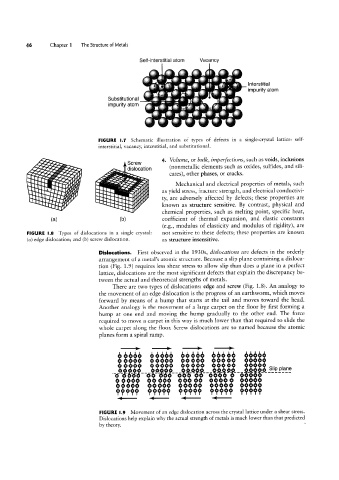
46 Chapter 1 The Structure of Metals
Self-interstitial atom is Vacancy
Substltutlonal lnterstitial
is
QE
F
FIGURE l.7 Schematic illustration of types of defects in a single-crystal lattice: self-
interstitial, vacancy, interstitial, and substitutional.
u
ll 'Q 4 ' $ "` 4. Volume, or bulk, imperfections, such as voids, inclusions
Screw
O
(nonmetallic elements such as oxides, sulfides, and sili-
_.,~ d|sIocat|on
cates), other phases, or cracks.
O €’i4;‘7f~
~
*®~.! 5
'Q
I
0Q0
I
Mechanical and electrical properties of metals, such
"npr,
I- '||,I
ld' li" /1/0
\\=\.'f-.g~¢//ag I lu ‘~ ~ as yield stress, fracture strength, and electrical conductivi-
~.}ll "5 f;
||=l|.I////; _ ll 'lu 'Hu'
n W5
I" I | l| l I f v' _=l_=||-=l|||¢s| ty, are adversely affected by defects; these properties are
lll====EE¢:|
"====iW known as structure sensitive. By contrast, physical and
chemical properties, such as melting point, specific heat,
coefficient of thermal expansion, and elastic constants
<a> <b>
(e.g., modulus of elasticity and modulus of rigidity), are
FIGURE I.8 Types of dislocations in a single crystal: not sensitive to these defects; these properties are known
(a) edge dislocation; and (b) screw dislocation. as structure insensitive.
Dislocations. First observed in the 19305, dislocations are defects in the orderly
arrangement of a metal’s atomic structure. Because a slip plane containing a disloca-
tion (Fig. 1.9) requires less shear stress to allow slip than does a plane in a perfect
lattice, dislocations are the most significant defects that explain the discrepancy be-
tween the actual and theoretical strengths of metals.
There are two types of dislocations: edge and screw (Fig. 1.8). An analogy to
the movement of an edge dislocation is the progress of an earthworm, which moves
forward by means of a hump that starts at the tail and moves toward the head.
Another analogy is the movement of a large carpet on the floor by first forming a
hump at one end and moving the hump gradually to the other end. The force
required to move a carpet in this way is much lower than that required to slide the
.ttll
whole carpet along the floor. Screw dislocations are so named because the atomic
4-l(-1-4141
FIGURE |.9 Movement of an edge dislocation across the crystal lattice under a shear stress.
Dislocations help explain why the actual strength of metals is much lower than that predicted
by theory.