Page 236 - Materials Science and Engineering An Introduction
P. 236
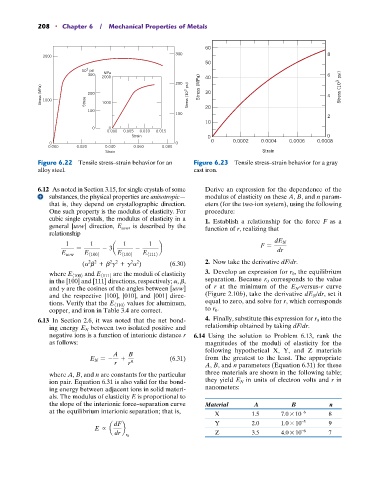
208 • Chapter 6 / Mechanical Properties of Metals
60
300 8
2000
50
3
10 psi MPa
300 6
2000 40
200 Stress (MPa) Stress (10 3 psi)
Stress (MPa) 1000 Stress 200 1000 Stress (10 3 psi) 30 4
100 20
100
2
10
0 0
0.000 0.005 0.010 0.015
Strain 0 0
0 0.0002 0.0004 0.0006 0.0008
0 0
0.000 0.020 0.040 0.060 0.080
Strain Strain
Figure 6.22 Tensile stress–strain behavior for an Figure 6.23 Tensile stress–strain behavior for a gray
alloy steel. cast iron.
6.12 As noted in Section 3.15, for single crystals of some Derive an expression for the dependence of the
substances, the physical properties are anisotropic— modulus of elasticity on these A, B, and n param-
that is, they depend on crystallographic direction. eters (for the two-ion system), using the following
One such property is the modulus of elasticity. For procedure:
cubic single crystals, the modulus of elasticity in a as a
general [uvw] direction, E uvw , is described by the 1. Establish a relationship for the force F
function of r, realizing that
relationship
1 1 1 1 F = dE N
= - 3a - b dr
E uvw E 81009 E 81009 E 81119
2 2
2 2
(a b + b g + g a ) (6.30) 2. Now take the derivative dF/dr.
2 2
where E 81009 and E 81119 are the moduli of elasticity 3. Develop an expression for r 0 , the equilibrium
in the [100] and [111] directions, respectively; a, b, separation. Because r 0 corresponds to the value
and g are the cosines of the angles between [uvw] of r at the minimum of the E N -versus-r curve
and the respective [100], [010], and [001] direc- (Figure 2.10b), take the derivative dE N /dr, set it
tions. Verify that the E 81109 values for aluminum, equal to zero, and solve for r, which corresponds
copper, and iron in Table 3.4 are correct. to r 0 .
6.13 In Section 2.6, it was noted that the net bond- 4. Finally, substitute this expression for r 0 into the
ing energy E N between two isolated positive and relationship obtained by taking dF/dr.
negative ions is a function of interionic distance r 6.14 Using the solution to Problem 6.13, rank the
as follows: magnitudes of the moduli of elasticity for the
following hypothetical X, Y, and Z materials
A B
E N = - + (6.31) from the greatest to the least. The appropriate
r r n A, B, and n parameters (Equation 6.31) for these
where A, B, and n are constants for the particular three materials are shown in the following table;
ion pair. Equation 6.31 is also valid for the bond- they yield E N in units of electron volts and r in
ing energy between adjacent ions in solid materi- nanometers:
als. The modulus of elasticity E is proportional to
the slope of the interionic force–separation curve Material A B n
at the equilibrium interionic separation; that is, X 1.5 7.0 * 10 -6 8
dF Y 2.0 1.0 * 10 -5 9
E a b -6
dr Z 3.5 4.0 * 10 7
r 0