Page 65 - Materials Science and Engineering An Introduction
P. 65
2.6 Primary Interatomic Bonds • 37
2p
2s
Energy
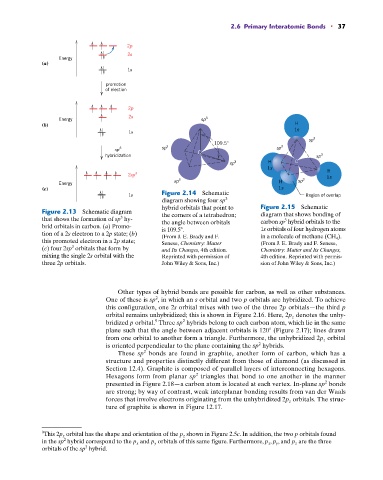
(a)
1s
promotion
of election
2p
2s
Energy sp 3
(b) H
1s
1s
3
sp
109.5° 3
sp 3 sp 3 sp
hybridization C sp 3
sp 3 H C
1s
H
2sp 3 1s
sp 3 H sp 3
Energy
(c) 1s
1s Figure 2.14 Schematic Region of overlap
3
diagram showing four sp
hybrid orbitals that point to Figure 2.15 Schematic
Figure 2.13 Schematic diagram the corners of a tetrahedron; diagram that shows bonding of
3
that shows the formation of sp hy- the angle between orbitals carbon sp hybrid orbitals to the
3
brid orbitals in carbon. (a) Promo- is 109.5 . 1s orbitals of four hydrogen atoms
tion of a 2s electron to a 2p state; (b) (From J. E. Brady and F. in a molecule of methane (CH 4 ).
this promoted electron in a 2p state; Senese, Chemistry: Matter (From J. E. Brady and F. Senese,
3
(c) four 2sp orbitals that form by and Its Changes, 4th edition. Chemistry: Matter and Its Changes,
mixing the single 2s orbital with the Reprinted with permission of 4th edition. Reprinted with permis-
three 2p orbitals. John Wiley & Sons, Inc.) sion of John Wiley & Sons, Inc.)
Other types of hybrid bonds are possible for carbon, as well as other substances.
2
One of these is sp , in which an s orbital and two p orbitals are hybridized. To achieve
this configuration, one 2s orbital mixes with two of the three 2p orbitals—the third p
orbital remains unhybridized; this is shown in Figure 2.16. Here, 2p z denotes the unhy-
2
9
bridized p orbital. Three sp hybrids belong to each carbon atom, which lie in the same
plane such that the angle between adjacent orbitals is 120 (Figure 2.17); lines drawn
from one orbital to another form a triangle. Furthermore, the unhybridized 2p z orbital
2
is oriented perpendicular to the plane containing the sp hybrids.
These sp 2 bonds are found in graphite, another form of carbon, which has a
structure and properties distinctly different from those of diamond (as discussed in
Section 12.4). Graphite is composed of parallel layers of interconnecting hexagons.
Hexagons form from planar sp 2 triangles that bond to one another in the manner
2
presented in Figure 2.18—a carbon atom is located at each vertex. In-plane sp bonds
are strong; by way of contrast, weak interplanar bonding results from van der Waals
forces that involve electrons originating from the unhybridized 2p z orbitals. The struc-
ture of graphite is shown in Figure 12.17.
9 This 2p z orbital has the shape and orientation of the p z shown in Figure 2.5c. In addition, the two p orbitals found
2
in the sp hybrid correspond to the p x and p y orbitals of this same figure. Furthermore, p x , p y , and p z are the three
3
orbitals of the sp hybrid.