Page 68 - Materials Science and Engineering An Introduction
P. 68
40 • Chapter 2 / Atomic Structure and Interatomic Bonding
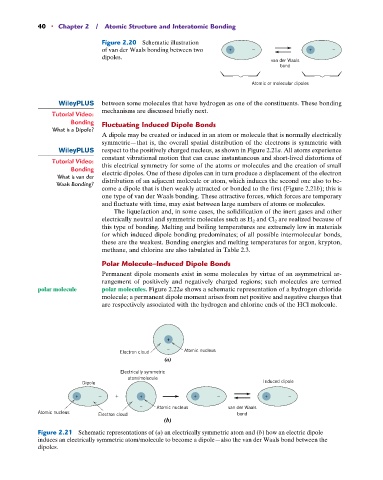
Figure 2.20 Schematic illustration
of van der Waals bonding between two + – – + ––
dipoles.
van der Waals
bond
Atomic or molecular dipoles
between some molecules that have hydrogen as one of the constituents. These bonding
mechanisms are discussed briefly next.
Tutorial Video:
Bonding Fluctuating Induced Dipole Bonds
What is a Dipole?
A dipole may be created or induced in an atom or molecule that is normally electrically
symmetric—that is, the overall spatial distribution of the electrons is symmetric with
respect to the positively charged nucleus, as shown in Figure 2.21a. All atoms experience
constant vibrational motion that can cause instantaneous and short-lived distortions of
Tutorial Video: this electrical symmetry for some of the atoms or molecules and the creation of small
Bonding electric dipoles. One of these dipoles can in turn produce a displacement of the electron
What is van der distribution of an adjacent molecule or atom, which induces the second one also to be-
Waals Bonding?
come a dipole that is then weakly attracted or bonded to the first (Figure 2.21b); this is
one type of van der Waals bonding. These attractive forces, which forces are temporary
and fluctuate with time, may exist between large numbers of atoms or molecules.
The liquefaction and, in some cases, the solidification of the inert gases and other
electrically neutral and symmetric molecules such as H 2 and Cl 2 are realized because of
this type of bonding. Melting and boiling temperatures are extremely low in materials
for which induced dipole bonding predominates; of all possible intermolecular bonds,
these are the weakest. Bonding energies and melting temperatures for argon, krypton,
methane, and chlorine are also tabulated in Table 2.3.
Polar Molecule–Induced Dipole Bonds
Permanent dipole moments exist in some molecules by virtue of an asymmetrical ar-
rangement of positively and negatively charged regions; such molecules are termed
polar molecule polar molecules. Figure 2.22a shows a schematic representation of a hydrogen chloride
molecule; a permanent dipole moment arises from net positive and negative charges that
are respectively associated with the hydrogen and chlorine ends of the HCl molecule.
+
–
Electron cloud Atomic nucleus
(a)
Electrically symmetric
atom/molecule
Dipole Induced dipole
+ –– + + + –– + – –
– Atomic nucleus van der Waals
Atomic nucleus
Electron cloud bond
(b)
Figure 2.21 Schematic representations of (a) an electrically symmetric atom and (b) how an electric dipole
induces an electrically symmetric atom/molecule to become a dipole—also the van der Waals bond between the
dipoles.