Page 82 - Materials Science and Engineering An Introduction
P. 82
54 • Chapter 3 / The Structure of Crystalline Solids
chosen to represent the symmetry of the crystal structure, wherein all the atom positions
in the crystal may be generated by translations of the unit cell integral distances along
each of its edges. Thus, the unit cell is the basic structural unit or building block of the
crystal structure and defines the crystal structure by virtue of its geometry and the atom
positions within. Convenience usually dictates that parallelepiped corners coincide with
centers of the hard-sphere atoms. Furthermore, more than a single unit cell may be cho-
sen for a particular crystal structure; however, we generally use the unit cell having the
highest level of geometrical symmetry.
3.4 METALLIC CRYSTAL STRUCTURES
The atomic bonding in this group of materials is metallic and thus nondirectional in
nature. Consequently, there are minimal restrictions as to the number and position
of nearest-neighbor atoms; this leads to relatively large numbers of nearest neighbors
and dense atomic packings for most metallic crystal structures. Also, for metals, when
we use the hard-sphere model for the crystal structure, each sphere represents an ion
core. Table 3.1 presents the atomic radii for a number of metals. Three relatively simple
crystal structures are found for most of the common metals: face-centered cubic, body-
centered cubic, and hexagonal close-packed.
The Face-Centered Cubic Crystal Structure
The crystal structure found for many metals has a unit cell of cubic geometry, with at-
oms located at each of the corners and the centers of all the cube faces. It is aptly called
face-centered cubic the face-centered cubic (FCC) crystal structure. Some of the familiar metals having this
(FCC) crystal structure are copper, aluminum, silver, and gold (see also Table 3.1). Figure 3.1a
shows a hard-sphere model for the FCC unit cell, whereas in Figure 3.1b the atom cent-
ers are represented by small circles to provide a better perspective on atom positions.
The aggregate of atoms in Figure 3.1c represents a section of crystal consisting of many
FCC unit cells. These spheres or ion cores touch one another across a face diagonal; the
cube edge length a and the atomic radius R are related through
Unit cell edge length
for face-centered a = 2R12 (3.1)
cubic
This result is obtained in Example Problem 3.1.
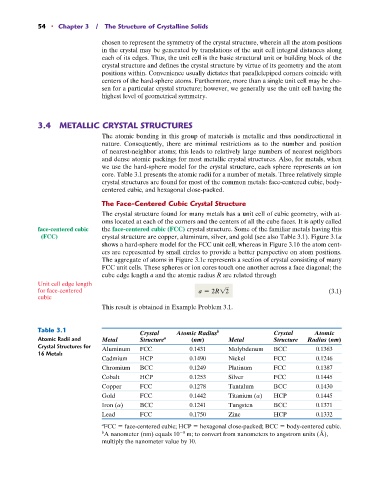
Table 3.1 b
Crystal Atomic Radius Crystal Atomic
Atomic Radii and Metal Structure a (nm) Metal Structure Radius (nm)
Crystal Structures for Aluminum FCC 0.1431 Molybdenum BCC 0.1363
16 Metals
Cadmium HCP 0.1490 Nickel FCC 0.1246
Chromium BCC 0.1249 Platinum FCC 0.1387
Cobalt HCP 0.1253 Silver FCC 0.1445
Copper FCC 0.1278 Tantalum BCC 0.1430
Gold FCC 0.1442 Titanium (a) HCP 0.1445
Iron (a) BCC 0.1241 Tungsten BCC 0.1371
Lead FCC 0.1750 Zinc HCP 0.1332
FCC face-centered cubic; HCP hexagonal close-packed; BCC body-centered cubic.
a
9
b A nanometer (nm) equals 10 m; to convert from nanometers to angstrom units (Å),
multiply the nanometer value by 10.