Page 111 - Materials Chemistry, Second Edition
P. 111
98 2 Solid-State Chemistry
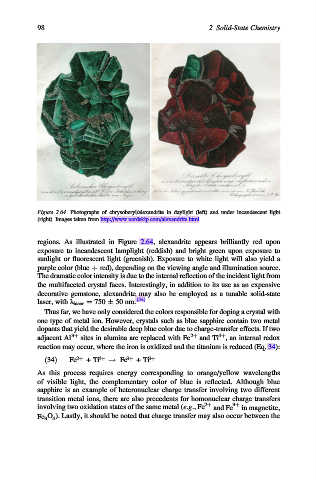
Figure 2.64. Photographs of chrysoberyl/alexandrite in daylight (left) and under incandescent light
(right). Images taken from http://www.nordskip.com/alexandrite.html.
regions. As illustrated in Figure 2.64, alexandrite appears brilliantly red upon
exposure to incandescent lamplight (reddish) and bright green upon exposure to
sunlight or fluorescent light (greenish). Exposure to white light will also yield a
purple color (blue þ red), depending on the viewing angle and illumination source.
The dramatic color intensity is due to the internal reflection of the incident light from
the multifaceted crystal faces. Interestingly, in addition to its use as an expensive
decorative gemstone, alexandrite may also be employed as a tunable solid-state
laser, with l laser ¼ 750 50 nm. [54]
Thus far, we have only considered the colors responsible for doping a crystal with
one type of metal ion. However, crystals such as blue sapphire contain two metal
dopants that yield the desirable deep blue color due to charge-transfer effects. If two
4þ
adjacent Al 3þ sites in alumina are replaced with Fe 2þ and Ti , an internal redox
reaction may occur, where the iron is oxidized and the titanium is reduced (Eq. 34):
ð34Þ Fe 2þ +Ti 4þ ! Fe 3þ +Ti 3þ
As this process requires energy corresponding to orange/yellow wavelengths
of visible light, the complementary color of blue is reflected. Although blue
sapphire is an example of heteronuclear charge transfer involving two different
transition metal ions, there are also precedents for homonuclear charge transfers
involving two oxidation states of the same metal (e.g.,Fe 2þ and Fe 3þ in magnetite,
Fe 3 O 4 ). Lastly, it should be noted that charge transfer may also occur between the