Page 107 - Materials Chemistry, Second Edition
P. 107
94 2 Solid-State Chemistry
and dopants that give rise to their characteristic colors. Whereas crystals of pure
corundum (a-alumina) are colorless, a small amount (<1%) of chromium doping
yields the familiar reddish/pink color. This color change is only possible if the
periodic framework of the crystal is altered, through the incorporation of additional
3þ
dopant atoms/ions or vacancies in the lattice. For ruby, a transition metal ion, Cr ,
replaces Al 3þ yielding electronic d–d transitions that were unattainable for the
original main-group ion.
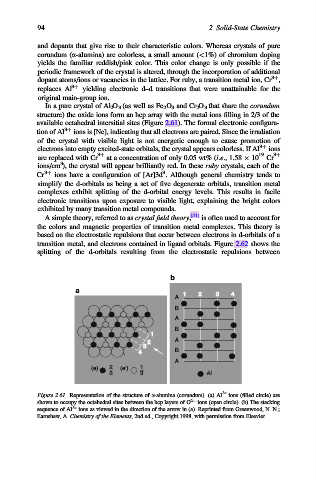
In a pure crystal of Al 2 O 3 (as well as Fe 2 O 3 and Cr 2 O 3 that share the corundum
structure) the oxide ions form an hcp array with the metal ions filling in 2/3 of the
available octahedral intersitial sites (Figure 2.61). The formal electronic configura-
tion of Al 3þ ions is [Ne], indicating that all electrons are paired. Since the irradiation
of the crystal with visible light is not energetic enough to cause promotion of
electrons into empty excited-state orbitals, the crystal appears colorless. If Al 3þ ions
are replaced with Cr 3þ at a concentration of only 0.05 wt% (i.e., 1.58 10 19 Cr 3þ
3
ions/cm ), the crystal will appear brilliantly red. In these ruby crystals, each of the
3
Cr 3þ ions have a configuration of [Ar]3d . Although general chemistry tends to
simplify the d-orbitals as being a set of five degenerate orbitals, transition metal
complexes exhibit splitting of the d-orbital energy levels. This results in facile
electronic transitions upon exposure to visible light, explaining the bright colors
exhibited by many transition metal compounds.
[51]
A simple theory, referred to as crystal field theory, is often used to account for
the colors and magnetic properties of transition metal complexes. This theory is
based on the electrostatic repulsions that occur between electrons in d-orbitals of a
transition metal, and electrons contained in ligand orbitals. Figure 2.62 shows the
splitting of the d-orbitals resulting from the electrostatic repulsions between
b
a
A 1 2 3 4
B
A
B
1
2 A
3
4 B
A
(e) 2 (e') 1
3 3 Al
Figure 2.61. Representation of the structure of a-alumina (corundum). (a) Al 3+ ions (filled circle) are
shown to occupy the octahedral sites between the hcp layers of O 2 ions (open circle). (b) The stacking
sequence of Al 3+ ions as viewed in the direction of the arrow in (a). Reprinted from Greenwood, N. N.;
Earnshaw, A. Chemistry of the Elements, 2nd ed., Copyright 1998, with permission from Elsevier.