Page 102 - Materials Chemistry, Second Edition
P. 102
89
2.3. The Crystalline State
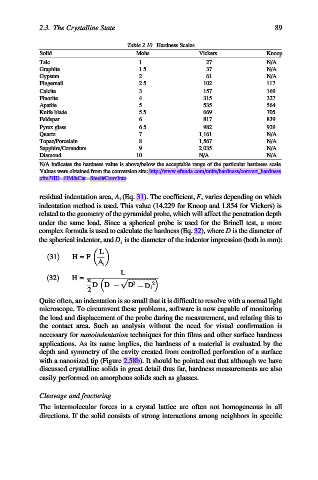
Table 2.10. Hardness Scales
Solid Mohs Vickers Knoop
Talc 1 27 N/A
Graphite 1.5 37 N/A
Gypsum 2 61 N/A
Fingernail 2.5 102 117
Calcite 3 157 169
Fluorite 4 315 327
Apatite 5 535 564
Knife blade 5.5 669 705
Feldspar 6 817 839
Pyrex glass 6.5 982 929
Quartz 7 1,161 N/A
Topaz/Porcelain 8 1,567 N/A
Sapphire/Corundum 9 2,035 N/A
Diamond 10 N/A N/A
N/A indicates the hardness value is above/below the acceptable range of the particular hardness scale.
Values were obtained from the conversion site: http://www.efunda.com/units/hardness/convert_hardness.
cfm?HD¼HM&Cat¼Steel#ConvInto.
residual indentation area, A r (Eq. 31). The coefficient, F, varies depending on which
indentation method is used. This value (14.229 for Knoop and 1.854 for Vickers) is
related to the geometry of the pyramidal probe, which will affect the penetration depth
under the same load. Since a spherical probe is used for the Brinell test, a more
complex formula is used to calculate the hardness (Eq. 32), where D is the diameter of
the spherical indentor, and D i is the diameter of the indentor impression (both in mm):
L
ð31Þ H=F
A r
L
ð32Þ H= p p ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
2
DD D D i 2
2
Quite often, an indentation is so small that it is difficult to resolve with a normal light
microscope. To circumvent these problems, software is now capable of monitoring
the load and displacement of the probe during the measurement, and relating this to
the contact area. Such an analysis without the need for visual confirmation is
necessary for nanoindentation techniques for thin films and other surface hardness
applications. As its name implies, the hardness of a material is evaluated by the
depth and symmetry of the cavity created from controlled perforation of a surface
with a nanosized tip (Figure 2.58b). It should be pointed out that although we have
discussed crystalline solids in great detail thus far, hardness measurements are also
easily performed on amorphous solids such as glasses.
Cleavage and fracturing
The intermolecular forces in a crystal lattice are often not homogeneous in all
directions. If the solid consists of strong interactions among neighbors in specific