Page 97 - Materials Chemistry, Second Edition
P. 97
84 2 Solid-State Chemistry
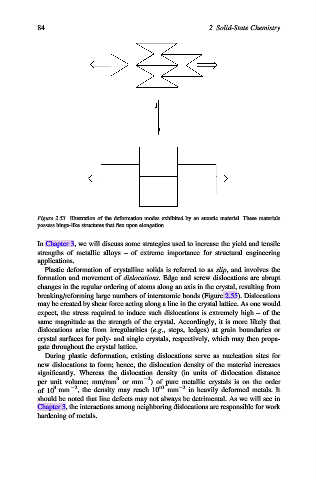
Figure 2.53. Illustration of the deformation modes exhibited by an auxetic material. These materials
possess hinge-like structures that flex upon elongation.
In Chapter 3, we will discuss some strategies used to increase the yield and tensile
strengths of metallic alloys – of extreme importance for structural engineering
applications.
Plastic deformation of crystalline solids is referred to as slip, and involves the
formation and movement of dislocations. Edge and screw dislocations are abrupt
changes in the regular ordering of atoms along an axis in the crystal, resulting from
breaking/reforming large numbers of interatomic bonds (Figure 2.55). Dislocations
may be created by shear force acting along a line in the crystal lattice. As one would
expect, the stress required to induce such dislocations is extremely high – of the
same magnitude as the strength of the crystal. Accordingly, it is more likely that
dislocations arise from irregularities (e.g., steps, ledges) at grain boundaries or
crystal surfaces for poly- and single crystals, respectively, which may then propa-
gate throughout the crystal lattice.
During plastic deformation, existing dislocations serve as nucleation sites for
new dislocations to form; hence, the dislocation density of the material increases
significantly. Whereas the dislocation density (in units of dislocation distance
2
3
per unit volume; mm/mm or mm ) of pure metallic crystals is on the order
3
2
of 10 mm , the density may reach 10 10 mm 2 in heavily deformed metals. It
should be noted that line defects may not always be detrimental. As we will see in
Chapter 3, the interactions among neighboring dislocations are responsible for work
hardening of metals.