Page 101 - Materials Chemistry, Second Edition
P. 101
88 2 Solid-State Chemistry
+
+
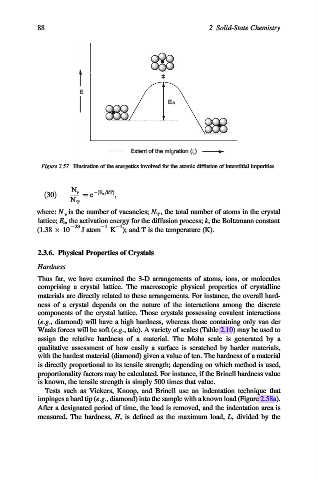
E
E a
Extent of the migration (z)
Figure 2.57. Illustration of the energetics involved for the atomic diffusion of interstitial impurities.
N v
ð30Þ ¼ e ðE a =kTÞ ;
N T
where: N v is the number of vacancies; N T , the total number of atoms in the crystal
lattice; E a , the activation energy for the diffusion process; k, the Boltzmann constant
1
(1.38 10 23 J atom 1 K ); and T is the temperature (K).
2.3.6. Physical Properties of Crystals
Hardness
Thus far, we have examined the 3-D arrangements of atoms, ions, or molecules
comprising a crystal lattice. The macroscopic physical properties of crystalline
materials are directly related to these arrangements. For instance, the overall hard-
ness of a crystal depends on the nature of the interactions among the discrete
components of the crystal lattice. Those crystals possessing covalent interactions
(e.g., diamond) will have a high hardness, whereas those containing only van der
Waals forces will be soft (e.g., talc). A variety of scales (Table 2.10) may be used to
assign the relative hardness of a material. The Mohs scale is generated by a
qualitative assessment of how easily a surface is scratched by harder materials,
with the hardest material (diamond) given a value of ten. The hardness of a material
is directly proportional to its tensile strength; depending on which method is used,
proportionality factors may be calculated. For instance, if the Brinell hardness value
is known, the tensile strength is simply 500 times that value.
Tests such as Vickers, Knoop, and Brinell use an indentation technique that
impinges a hard tip (e.g., diamond) into the sample with a known load (Figure 2.58a).
After a designated period of time, the load is removed, and the indentation area is
measured. The hardness, H, is defined as the maximum load, L, divided by the