Page 100 - Materials Chemistry, Second Edition
P. 100
87
2.3. The Crystalline State
Table 2.9. Slip Systems for BCC, HCP and FCC Crystals
Crystal Slip plane Slip direction
Body-centered cubic (BCC) {110} <111>
{211} <111>
{321} <111>
Hexagonal close-packed (HCP) {0001} <1120>
{1010} <1120>
{1011} <1120>
Face-centered cubic (FCC) {111} <110>
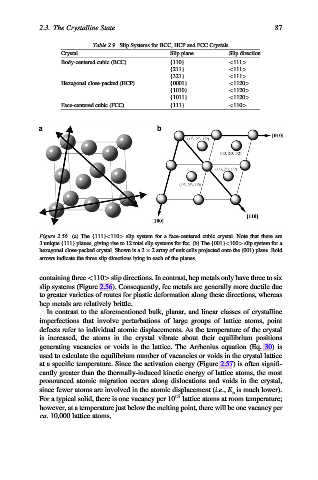
Figure 2.56. (a) The {111}<110> slip system for a face-centered cubic crystal. Note that there are
3 unique {111} planes, giving rise to 12 total slip systems for fcc. (b) The {001}<100> slip system for a
hexagonal close-packed crystal. Shown is a 2 2 array of unit cells projected onto the (001) plane. Bold
arrows indicate the three slip directions lying in each of the planes.
containing three <110> slip directions. In contrast, hcp metals only have three to six
slip systems (Figure 2.56). Consequently, fcc metals are generally more ductile due
to greater varieties of routes for plastic deformation along these directions, whereas
hcp metals are relatively brittle.
In contrast to the aforementioned bulk, planar, and linear classes of crystalline
imperfections that involve perturbations of large groups of lattice atoms, point
defects refer to individual atomic displacements. As the temperature of the crystal
is increased, the atoms in the crystal vibrate about their equilibrium positions
generating vacancies or voids in the lattice. The Arrhenius equation (Eq. 30)is
used to calculate the equilibrium number of vacancies or voids in the crystal lattice
at a specific temperature. Since the activation energy (Figure 2.57) is often signifi-
cantly greater than the thermally-induced kinetic energy of lattice atoms, the most
pronounced atomic migration occurs along dislocations and voids in the crystal,
since fewer atoms are involved in the atomic displacement (i.e., E a is much lower).
For a typical solid, there is one vacancy per 10 15 lattice atoms at room temperature;
however, at a temperature just below the melting point, there will be one vacancy per
ca. 10,000 lattice atoms.