Page 150 - Materials Chemistry, Second Edition
P. 150
137
2.4. The Amorphous State
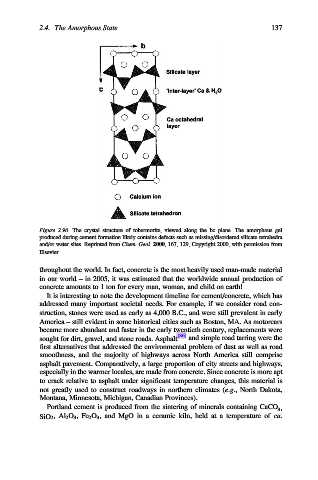
Figure 2.96. The crystal structure of tobermorite, viewed along the bc plane. The amorphous gel
produced during cement formation likely contains defects such as missing/disordered silicate tetrahedra
and/or water sites. Reprinted from Chem. Geol. 2000, 167, 129, Copyright 2000, with permission from
Elsevier.
throughout the world. In fact, concrete is the most heavily used man-made material
in our world – in 2005, it was estimated that the worldwide annual production of
concrete amounts to 1 ton for every man, woman, and child on earth!
It is interesting to note the development timeline for cement/concrete, which has
addressed many important societal needs. For example, if we consider road con-
struction, stones were used as early as 4,000 B.C., and were still prevalent in early
America – still evident in some historical cities such as Boston, MA. As motorcars
became more abundant and faster in the early twentieth century, replacements were
sought for dirt, gravel, and stone roads. Asphalt [86] and simple road tarring were the
first alternatives that addressed the environmental problem of dust as well as road
smoothness, and the majority of highways across North America still comprise
asphalt pavement. Comparatively, a large proportion of city streets and highways,
especially in the warmer locales, are made from concrete. Since concrete is more apt
to crack relative to asphalt under significant temperature changes, this material is
not greatly used to construct roadways in northern climates (e.g., North Dakota,
Montana, Minnesota, Michigan, Canadian Provinces).
Portland cement is produced from the sintering of minerals containing CaCO 3 ,
SiO 2 ,Al 2 O 3 ,Fe 2 O 3 , and MgO in a ceramic kiln, held at a temperature of ca.