Page 151 - Materials Chemistry, Second Edition
P. 151
138 2 Solid-State Chemistry
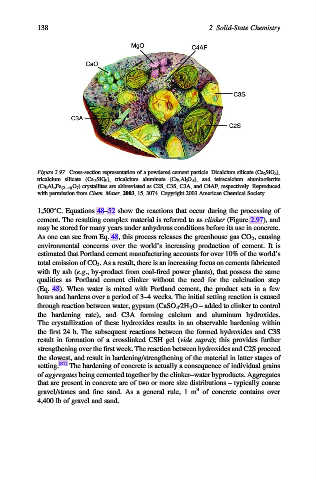
Figure 2.97. Cross-section representation of a powdered cement particle. Dicalcium silicate (Ca 2 SiO 4 ),
tricalcium silicate (Ca 3 SiO 5 ), tricalcium aluminate (Ca 3 Al 2 O 6 ), and tetracalcium aluminoferrite
(Ca 4 Al n Fe (2 n) O 7 ) crystallites are abbreviated as C2S, C3S, C3A, and C4AF, respectively. Reproduced
with permission from Chem. Mater. 2003, 15, 3074. Copyright 2003 American Chemical Society.
1,500 C. Equations 48–52 show the reactions that occur during the processing of
cement. The resulting complex material is referred to as clinker (Figure 2.97), and
may be stored for many years under anhydrous conditions before its use in concrete.
As one can see from Eq. 48, this process releases the greenhouse gas CO 2 , causing
environmental concerns over the world’s increasing production of cement. It is
estimated that Portland cement manufacturing accounts for over 10% of the world’s
total emission of CO 2 . As a result, there is an increasing focus on cements fabricated
with fly ash (e.g., by-product from coal-fired power plants), that possess the same
qualities as Portland cement clinker without the need for the calcination step
(Eq. 48). When water is mixed with Portland cement, the product sets in a few
hours and hardens over a period of 3–4 weeks. The initial setting reaction is caused
through reaction between water, gypsum (CaSO 4 ·2H 2 O – added to clinker to control
the hardening rate), and C3A forming calcium and aluminum hydroxides.
The crystallization of these hydroxides results in an observable hardening within
the first 24 h. The subsequent reactions between the formed hydroxides and C3S
result in formation of a crosslinked CSH gel (vide supra); this provides further
strengthening over the first week. The reaction between hydroxides and C2S proceed
the slowest, and result in hardening/strengthening of the material in latter stages of
setting. [87] The hardening of concrete is actually a consequence of individual grains
of aggregates being cemented together by the clinker–water byproducts. Aggregates
that are present in concrete are of two or more size distributions – typically coarse
3
gravel/stones and fine sand. As a general rule, 1 m of concrete contains over
4,400 lb of gravel and sand.