Page 194 - Materials Chemistry, Second Edition
P. 194
181
3.2. Metallic Structures and Properties
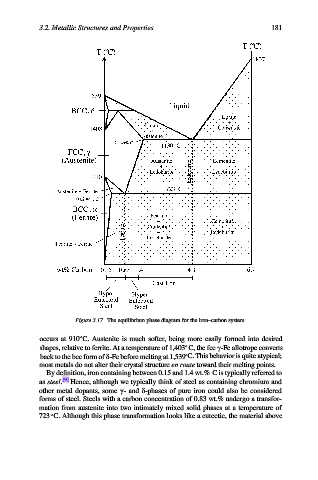
Figure 3.17. The equilibrium phase diagram for the iron–carbon system.
occurs at 910 C. Austenite is much softer, being more easily formed into desired
shapes, relative to ferrite. At a temperature of 1,403 C, the fcc g-Fe allotrope converts
back to the bcc form of d-Fe before melting at 1,539 C. This behavior is quite atypical;
most metals do not alter their crystal structure en route toward their melting points.
By definition, iron containing between 0.15 and 1.4 wt.% C is typically referred to
as steel. [9] Hence, although we typically think of steel as containing chromium and
other metal dopants, some g- and d-phases of pure iron could also be considered
forms of steel. Steels with a carbon concentration of 0.83 wt.% undergo a transfor-
mation from austenite into two intimately mixed solid phases at a temperature of
723 C. Although this phase transformation looks like a eutectic, the material above