Page 199 - Materials Chemistry, Second Edition
P. 199
186 3 Metals
determine the grain sizes; most commercial metals and alloys consist of individual
crystallites with diameters ranging from 10 to 100 mm, each corresponding to millions
of individual metal atoms. A decrease in the size of these microscopic particles results
in an increase in both strength and hardness of the bulk material. This can be
understood by the tighter packing of smaller spheres relative to larger ones, effectively
resisting atomic repositioning as a result of an external stress such as bending.
Heat treatment of iron alloys will affect the slip characteristics of the material
through changes in crystallite sizes. As the grain diameters become larger through
annealing, more grain boundaries will tend to form, resulting in a greater proclivity
for slip deformations. In general, as the grain size is decreased, the strength of the
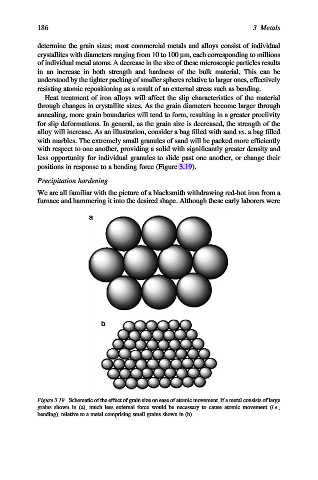
alloy will increase. As an illustration, consider a bag filled with sand vs. a bag filled
with marbles. The extremely small granules of sand will be packed more efficiently
with respect to one another, providing a solid with significantly greater density and
less opportunity for individual granules to slide past one another, or change their
positions in response to a bending force (Figure 3.19).
Precipitation hardening
We are all familiar with the picture of a blacksmith withdrawing red-hot iron from a
furnace and hammering it into the desired shape. Although these early laborers were
Figure 3.19. Schematic of the effect of grain size on ease of atomic movement. If a metal consists of large
grains shown in (a), much less external force would be necessary to cause atomic movement (i.e.,
bending), relative to a metal comprising small grains shown in (b).