Page 201 - Materials Chemistry, Second Edition
P. 201
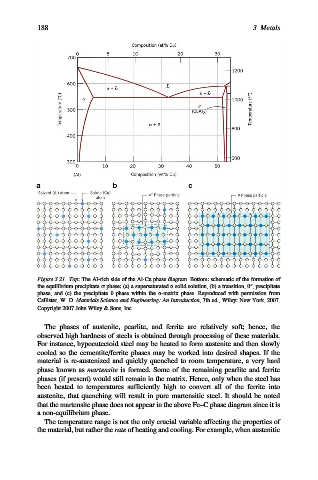
188 3 Metals
Figure 3.21. Top: The Al-rich side of the Al-Cu phase diagram. Bottom: schematic of the formation of
00
the equilibrium precipitate s phase: (a) a supersaturated a solid solution, (b) a transition, y , precipitate
phase, and (c) the precipitate y phase within the a-matrix phase. Reproduced with permission from
Callister, W. D. Materials Science and Engineering: An Introduction, 7th ed., Wiley: New York, 2007.
Copyright 2007 John Wiley & Sons, Inc.
The phases of austenite, pearlite, and ferrite are relatively soft; hence, the
observed high hardness of steels is obtained through processing of these materials.
For instance, hypoeutectoid steel may be heated to form austenite and then slowly
cooled so the cementite/ferrite phases may be worked into desired shapes. If the
material is re-austenized and quickly quenched to room temperature, a very hard
phase known as martensite is formed. Some of the remaining pearlite and ferrite
phases (if present) would still remain in the matrix. Hence, only when the steel has
been heated to temperatures sufficiently high to convert all of the ferrite into
austenite, that quenching will result in pure martensitic steel. It should be noted
that the martensite phase does not appear in the above Fe–C phase diagram since it is
a non-equilibrium phase.
The temperature range is not the only crucial variable affecting the properties of
the material, but rather the rate of heating and cooling. For example, when austenitic