Page 205 - Materials Chemistry, Second Edition
P. 205
192 3 Metals
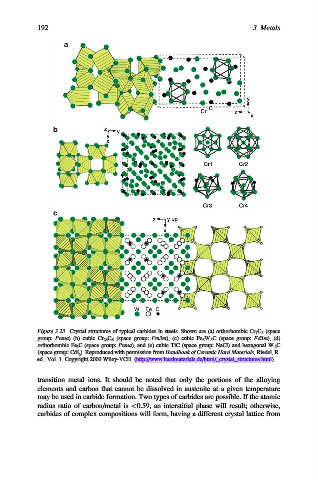
Figure 3.23. Crystal structures of typical carbides in steels. Shown are (a) orthorhombic Cr 7 C 3 (space
group: Pnma) (b) cubic Cr 23 C 6 (space group: Fm3m), (c) cubic Fe 3 W 3 C (space group: Fd3m), (d)
orthorhombic Fe 3 C (space group: Pnma), and (e) cubic TiC (space group: NaCl) and hexagonal W 2 C
(space group: CdI 2 ). Reproduced with permission from Handbook of Ceramic Hard Materials, Riedel, R.
ed., Vol. 1. Copyright 2000 Wiley-VCH. (http://www.hardmaterials.de/html/_crystal_structures.html).
transition metal ions. It should be noted that only the portions of the alloying
elements and carbon that cannot be dissolved in austenite at a given temperature
may be used in carbide formation. Two types of carbides are possible. If the atomic
radius ratio of carbon/metal is <0.59, an interstitial phase will result; otherwise,
carbides of complex compositions will form, having a different crystal lattice from