Page 219 - Materials Chemistry, Second Edition
P. 219
206 3 Metals
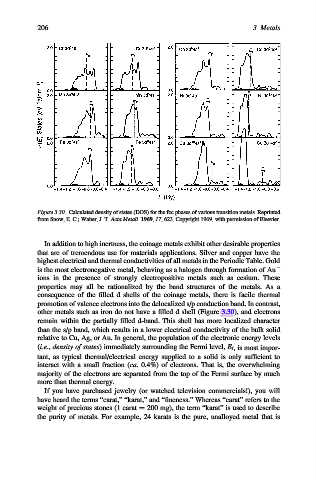
Figure 3.30. Calculated density of states (DOS) for the fcc phases of various transition metals. Reprinted
from Snow, E. C.; Waber, J. T. Acta Metall. 1969, 17, 623. Copyright 1969, with permission of Elsevier.
In addition to high inertness, the coinage metals exhibit other desirable properties
that are of tremendous use for materials applications. Silver and copper have the
highest electrical and thermal conductivities of all metals in the Periodic Table. Gold
is the most electronegative metal, behaving as a halogen through formation of Au
ions in the presence of strongly electropositive metals such as cesium. These
properties may all be rationalized by the band structures of the metals. As a
consequence of the filled d shells of the coinage metals, there is facile thermal
promotion of valence electrons into the delocalized s/p conduction band. In contrast,
other metals such as iron do not have a filled d shell (Figure 3.30), and electrons
remain within the partially filled d-band. This shell has more localized character
than the s/p band, which results in a lower electrical conductivity of the bulk solid
relative to Cu, Ag, or Au. In general, the population of the electronic energy levels
(i.e., density of states) immediately surrounding the Fermi level, E f , is most impor-
tant, as typical thermal/electrical energy supplied to a solid is only sufficient to
interact with a small fraction (ca. 0.4%) of electrons. That is, the overwhelming
majority of the electrons are separated from the top of the Fermi surface by much
more than thermal energy.
If you have purchased jewelry (or watched television commercials!), you will
have heard the terms “carat,” “karat,” and “fineness.” Whereas “carat” refers to the
weight of precious stones (1 carat ¼ 200 mg), the term “karat” is used to describe
the purity of metals. For example, 24 karats is the pure, unalloyed metal that is