Page 220 - Materials Chemistry, Second Edition
P. 220
207
3.2. Metallic Structures and Properties
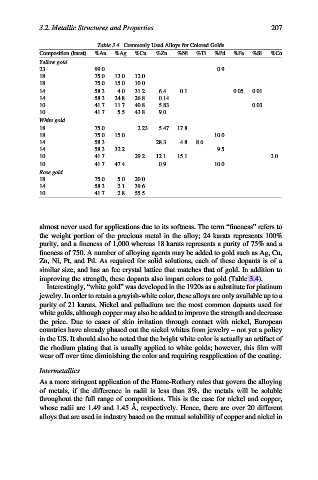
Table 3.4. Commonly Used Alloys for Colored Golds
Composition (karat) %Au %Ag %Cu %Zn %Ni %Ti %Pd %Fe %Si %Co
Yellow gold
23 99.0 0.9
18 75.0 13.0 12.0
18 75.0 15.0 10.0
14 58.3 4.0 31.2 6.4 0.1 0.05 0.01
14 58.3 24.8 26.8 0.14
10 41.7 11.7 40.8 5.83 0.03
10 41.7 5.5 43.8 9.0
White gold
18 75.0 2.23 5.47 17.8
18 75.0 15.0 10.0
14 58.3 28.3 4.8 8.6
14 58.3 32.2 9.5
10 41.7 29.2 12.1 15.1 2.0
10 41.7 47.4 0.9 10.0
Rose gold
18 75.0 5.0 20.0
14 58.3 2.1 39.6
10 41.7 2.8 55.5
almost never used for applications due to its softness. The term “fineness” refers to
the weight portion of the precious metal in the alloy; 24 karats represents 100%
purity, and a fineness of 1,000 whereas 18 karats represents a purity of 75% and a
fineness of 750. A number of alloying agents may be added to gold such as Ag, Cu,
Zn, Ni, Pt, and Pd. As required for solid solutions, each of these dopants is of a
similar size, and has an fcc crystal lattice that matches that of gold. In addition to
improving the strength, these dopants also impart colors to gold (Table 3.4).
Interestingly, “white gold” was developed in the 1920s as a substitute for platinum
jewelry. In order to retain a grayish-white color, these alloys are only available up to a
purity of 21 karats. Nickel and palladium are the most common dopants used for
white golds, although copper may also be added to improve the strength and decrease
the price. Due to cases of skin irritation through contact with nickel, European
countries have already phased out the nickel whites from jewelry – not yet a policy
in the US. It should also be noted that the bright white color is actually an artifact of
the rhodium plating that is usually applied to white golds; however, this film will
wear off over time diminishing the color and requiring reapplication of the coating.
Intermetallics
As a more stringent application of the Hume-Rothery rules that govern the alloying
of metals, if the difference in radii is less than 8%, the metals will be soluble
throughout the full range of compositions. This is the case for nickel and copper,
˚
whose radii are 1.49 and 1.45 A, respectively. Hence, there are over 20 different
alloys that are used in industry based on the mutual solubility of copper and nickel in